Vortioxetine5-HT receptors antagonist CAS# 508233-74-7 |
- Granisetron HCl
Catalog No.:BCC1060
CAS No.:107007-99-8
- SB 271046 hydrochloride
Catalog No.:BCC1924
CAS No.:209481-24-3
- Adoprazine
Catalog No.:BCC1329
CAS No.:222551-17-9
- SEA0400
Catalog No.:BCC1941
CAS No.:223104-29-8
- Tianeptine
Catalog No.:BCC1999
CAS No.:66981-73-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 508233-74-7 | SDF | Download SDF |
| PubChem ID | 9966051 | Appearance | Powder |
| Formula | C18H22N2S | M.Wt | 298.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (167.53 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
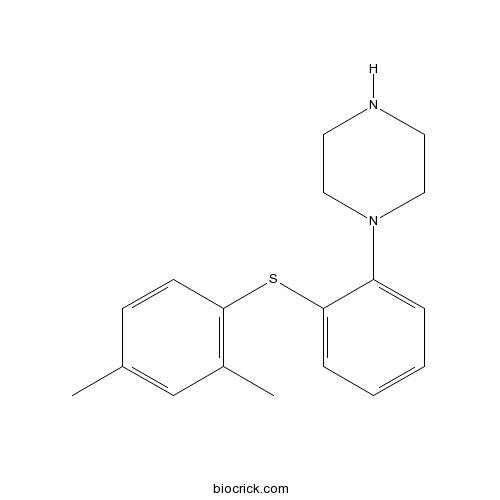
| Chemical Name | 1-[2-(2,4-dimethylphenyl)sulfanylphenyl]piperazine | ||
| SMILES | CC1=CC(=C(C=C1)SC2=CC=CC=C2N3CCNCC3)C | ||
| Standard InChIKey | YQNWZWMKLDQSAC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H22N2S/c1-14-7-8-17(15(2)13-14)21-18-6-4-3-5-16(18)20-11-9-19-10-12-20/h3-8,13,19H,9-12H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Vortioxetine is an antagonist of 5-HT3 receptor with IC50 value of 12 nM. | |||||
| Targets | 5-HT3 receptor | |||||
| IC50 | 12 nM | |||||

Vortioxetine Dilution Calculator

Vortioxetine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3506 mL | 16.7532 mL | 33.5064 mL | 67.0129 mL | 83.7661 mL |
| 5 mM | 0.6701 mL | 3.3506 mL | 6.7013 mL | 13.4026 mL | 16.7532 mL |
| 10 mM | 0.3351 mL | 1.6753 mL | 3.3506 mL | 6.7013 mL | 8.3766 mL |
| 50 mM | 0.067 mL | 0.3351 mL | 0.6701 mL | 1.3403 mL | 1.6753 mL |
| 100 mM | 0.0335 mL | 0.1675 mL | 0.3351 mL | 0.6701 mL | 0.8377 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Vortioxetine is a novel multimodal antidepressant currently under development for the treatment of MDD. The serotonergic system plays an important role in cognitive functions via various 5-HT receptors.
In vitro: Vortioxetine (Lu AA21004) was the lead compound, displaying high affinity for recombinant human 5-HT1A (Ki = 15 nM), 5-HT1B (Ki = 33 nM), 5-HT3A (Ki = 3.7 nM), 5-HT7 (Ki = 19 nM), and noradrenergic β1 (Ki = 46 nM) receptors, and SERT (Ki = 1.6 nM). Vortioxetine displayed antagonistic properties at 5-HT3A and 5-HT7 receptors, partial agonist properties at 5-HT1B receptors, agonistic properties at 5-HT1A receptors, and potent inhibition of SERT [1].
In vivo: In conscious rats, vortioxetine significantly increased extracellular 5-HT levels in the brain after acute and 3 days of treatment. Following the 3-day treatment (5 or 10 mg/kg/day) SERT occupancies were only 43% and 57%, respectively [1].
Clinical trial: Drugs were administered in the evening of 15 consecutive days. Vortioxetine did not cause cognitive or psychomotor impairment. However, mirtazapine impaired cognitive and psychomotor performance on day 2. Most of these effects disappeared after multiple doses of mirtazapine [2].
References:
[1] Bang-Andersen B, Ruhland T, Jørgensen M, Smith G, Frederiksen K, Jensen KG, Zhong H, Nielsen SM, Hogg S, Mørk A, Stensbøl TB. Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem. 2011;54(9):3206-21.
[2] Theunissen EL, Street D, Højer AM, Vermeeren A, van Oers A, Ramaekers JG. A randomized trial on the acute and steady-state effects of a new antidepressant, vortioxetine (Lu AA21004), on actual driving and cognition. Clin Pharmacol Ther. 2013;93(6):493-501.
- Ganoderic acid LM2
Catalog No.:BCN2442
CAS No.:508182-41-0
- Suchilactone
Catalog No.:BCN6752
CAS No.:50816-74-5
- Hastatoside
Catalog No.:BCN6898
CAS No.:50816-24-5
- Astrophylline
Catalog No.:BCN2151
CAS No.:5081-53-8
- Vasicinol
Catalog No.:BCN5812
CAS No.:5081-51-6
- Hellebrigenol
Catalog No.:BCN8238
CAS No.:508-79-2
- Convallatoxin
Catalog No.:BCC8155
CAS No.:508-75-8
- Rosenonolactone
Catalog No.:BCN5621
CAS No.:508-71-4
- Ouabagenin
Catalog No.:BCC8227
CAS No.:508-52-1
- N-acetylanonaine
Catalog No.:BCN2666
CAS No.:5894-74-6
- Glutinone
Catalog No.:BCN5618
CAS No.:508-09-8
- 13(18)-Oleanen-3-ol
Catalog No.:BCN5617
CAS No.:508-04-3
- 14,15-Didehydroisoeburnamine
Catalog No.:BCN5619
CAS No.:50838-11-4
- 1,3,5,6-Tetrahydroxyxanthone
Catalog No.:BCN3453
CAS No.:5084-31-1
- Friedelanol
Catalog No.:BCN5620
CAS No.:5085-72-3
- Tetramisole HCl
Catalog No.:BCC4735
CAS No.:5086-74-8
- 1,5,6-Trihydroxy-3-methoxyxanthone
Catalog No.:BCN8121
CAS No.:50868-52-5
- Neoliquiritin
Catalog No.:BCN6663
CAS No.:5088-75-5
- Liensinine diperchlorate
Catalog No.:BCN6336
CAS No.:5088-90-4
- 1,4-Bis(2-benzoxazolyl)naphthalene
Catalog No.:BCC8423
CAS No.:5089-22-5
- WY-14643 (Pirinixic Acid)
Catalog No.:BCC2265
CAS No.:50892-23-4
- 1-Acetyl-beta-carboline
Catalog No.:BCN3101
CAS No.:50892-83-6
- Gelsemine
Catalog No.:BCN5804
CAS No.:509-15-9
- Delsoline
Catalog No.:BCN5405
CAS No.:509-18-2
Acute dosing of vortioxetine strengthens event-related brain activity associated with engagement of attention and cognitive functioning in rats.[Pubmed:28366617]
Brain Res. 2017 Jun 1;1664:37-47.
Studies of the antidepressant Vortioxetine have demonstrated beneficial effects on cognitive dysfunction associated with depression. To elucidate how Vortioxetine modulates neuronal activity during cognitive processing we investigated the effects of Vortioxetine (3 and 10mg/kg) in rats performing an auditory oddball (deviant target) task. We investigated neuronal activity in target vs non-target tone responses in vehicle-treated animals using electroencephalographic (EEG) recordings. Furthermore, we characterized task performance and EEG changes in target tone responses of Vortioxetine vs controls. Quantification of event-related potentials (ERPs) was supplemented by analyses of spectral power and inter-trial phase-locking. The assessed brain regions included prelimbic cortex, the hippocampus, and thalamus. As compared to correct rejection of non-target tones, correct target tone responses elicited increased EEG power in all regions. Additionally, neuronal synchronization was increased in vehicle-treated rats during both early and late ERP responses to target tones. This indicates a significant consistency of local phases across trials during high attentional load. During early sensory processing, Vortioxetine increased both thalamic and frontal synchronized gamma band activity and EEG power in all brain regions measured. Finally, Vortioxetine increased the amplitude of late hippocampal P3-like ERPs, the rodent correlate of the human P300 ERP. These findings suggest differential effects of Vortioxetine during early sensory registration and late endogenous processing of auditory discrimination. Strengthened P3-like ERP response may relate to the pro-cognitive profile of Vortioxetine in rodents. Further investigations are warranted to explore the mechanism by which Vortioxetine increases network synchronization during attentive and cognitive processing.
The impact of antidepressant treatments on family functioning in adults with major depressive disorder: a post hoc comparison of vortioxetine and agomelatine.[Pubmed:28277865]
Curr Med Res Opin. 2017 Jun;33(6):1057-1066.
OBJECTIVE: There is limited research on the impact of antidepressant treatment on family functioning. This study examines the impact of Vortioxetine and agomelatine on family functioning using the Depression and Family Functioning Scale (DFFS). METHODS: The DFFS was included in REVIVE, a randomized, double-blind study of adults with major depressive disorder with inadequate response to antidepressant treatment who switched to Vortioxetine or agomelatine. The prespecified DFFS analyses were performed using change from baseline to weeks 8 and 12, analyzed by mixed models for repeated measurements by treatment groups. Post hoc analyses compared DFFS scores for remitters and nonremitters. Patients were stratified into quartiles using DFFS scores, and scores on other clinical outcome assessments were compared. RESULTS: Sizeable improvements in DFFS scores were observed from baseline to week 8 (-10.8, -7.9 for Vortioxetine and agomelatine, respectively), with further improvements at week 12 (-13.5, -11.0). Vortioxetine (n = 189) was superior to agomelatine (n = 187) by 2.9 DFFS points at week 8 (p < .01) and 2.5 points at week 12 (p < .05), and DFFS item-level improvements were also significantly greater for Vortioxetine for 8 of 15 DFFS items at week 8 and 7 items at week 12. At week 8, remitters (n = 142) and nonremitters (n = 233) differed by 11 DFFS points; at week 12, remitters (n = 183) and nonremitters (n = 121) differed by almost 12 DFFS points. Patients stratified into baseline DFFS quartiles showed trends on clinical outcomes such that better family functioning was associated with better functional status and depressive symptoms. CONCLUSIONS: Vortioxetine was significantly superior to agomelatine in terms of family functioning and partner relationships, as well as social functioning, health status, and depression symptoms at weeks 8 and 12. Depressed patients with impaired family functioning showed worse overall functioning, health status, and depression symptoms, suggesting that more attention should be given to family functioning of depressed patients.
The effect of vortioxetine on overall patient functioning in patients with major depressive disorder.[Pubmed:28293465]
Brain Behav. 2017 Feb 2;7(3):e00622.
BACKGROUND: The objectives of this meta-analysis of data from randomized, placebo-controlled studies were to assess the effect of Vortioxetine on overall functioning (primary) and functional remission (secondary) using the Sheehan Disability Scale (SDS) in adults with major depressive disorder (MDD). METHODS: Data from nine short-term (6/8 weeks) pivotal studies that included patient functioning assessments were included in this random-effects meta-analysis, which used aggregated study-level data for all therapeutic Vortioxetine doses and a mixed-effect model for repeated measures using the full analysis set. RESULTS: A total of 4,216 patients received >/=1 dose of study treatment (1,522 placebo, 2,694 Vortioxetine 5-20 mg/day). At study end, the meta-analysis showed improvement for Vortioxetine versus placebo (n = 911) in SDS total score (Vortioxetine 5 mg, n = 564, change from baseline versus placebo [Delta] -0.24, p = NS; 10 mg, n = 445, Delta -1.68, p Vortioxetine 10 mg (n = 170/573; odds ratio [OR] relative to placebo 1.7, p < .001) and 20 mg (n = 144/447; OR 1.6, p < .05), but not 5 mg (n = 207/757; OR 1.1, p = NS) or 15 mg (n = 92/295; OR 1.3, p = NS). CONCLUSION: Vortioxetine 5-20 mg for 6/8 weeks improved overall patient functioning in patients with MDD. Relative to placebo, Vortioxetine 10 and 20 mg demonstrated significant improvement in SDS total score and functional remission.
Pharmacokinetics and Safety of Vortioxetine in Pediatric Patients.[Pubmed:28333546]
J Child Adolesc Psychopharmacol. 2017 Aug;27(6):526-534.
OBJECTIVE: The primary objectives of this study were to evaluate the pharmacokinetics (PK) and tolerability of single and multiple doses of Vortioxetine in children and adolescents with a depressive or anxiety disorder and to provide supportive information for appropriate dosing regimens for pediatric clinical trials. METHODS: This prospective, open-label, multinational, multisite, multiple-dose trial enrolled 48 patients (children and adolescents; 1:1 ratio) divided into 8 cohorts (4 adolescent and 4 child), with each cohort including 6 patients. The cohorts in each age group were assigned to receive one of four dosing regimens: Vortioxetine 5, 10, 15, or 20 mg q.d. for 14 days. The total treatment period lasted 14-20 days with patients in the higher dose cohorts uptitrated over 2-6 days. Plasma samples for PK analysis were obtained on the first and last days of dosing. RESULTS: Among children and adolescents, respectively, 62% and 92% had depression and 58% and 33% had anxiety disorder. Comorbid attention-deficit/hyperactivity disorder (ADHD) was present in 50% of children and 38% of adolescents. After 14 days q.d. at the target dose, the PK of Vortioxetine concentrations was generally proportional to the dose in both age groups. Exposure, as assessed by maximum plasma concentrations and area under the plasma concentration-time curve from time 0 to 24 hours, was 30%-40% lower in adolescents than in children. There was no significant relationship between sex, height, or ADHD diagnosis and PK parameters. Most adverse events were mild in severity and consistent with those seen in adults. CONCLUSION: The results suggest that the dosages of Vortioxetine evaluated (5-20 mg q.d.; approved for treatment in adults) and the uptitration schedule used are appropriate for pediatric efficacy and safety trials.


