(±)-Palmitoylcarnitine chlorideProtein kinase C inhibitor CAS# 6865-14-1 |
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- MK-0752
Catalog No.:BCC2090
CAS No.:471905-41-6
- MRK 560
Catalog No.:BCC2345
CAS No.:677772-84-8
- PF-03084014
Catalog No.:BCC1848
CAS No.:865773-15-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6865-14-1 | SDF | Download SDF |
| PubChem ID | 363417 | Appearance | Powder |
| Formula | C23H46ClNO4 | M.Wt | 436.07 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in water | ||
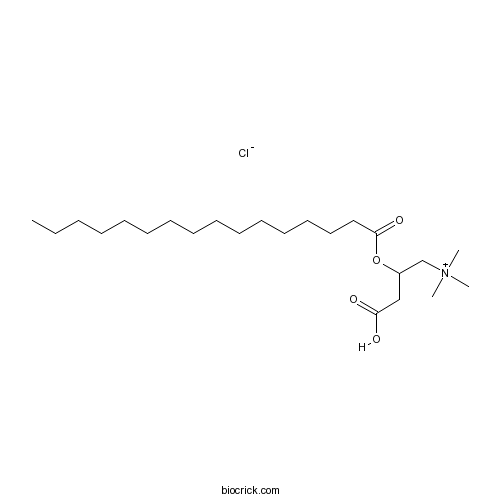
| Chemical Name | (3-carboxy-2-hexadecanoyloxypropyl)-trimethylazanium;chloride | ||
| SMILES | CCCCCCCCCCCCCCCC(=O)OC(CC(=O)O)C[N+](C)(C)C.[Cl-] | ||
| Standard InChIKey | GAMKNLFIHBMGQT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H45NO4.ClH/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-23(27)28-21(19-22(25)26)20-24(2,3)4;/h21H,5-20H2,1-4H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Intermediate in mitochondrial fatty acid oxidation. Has a wide range of biological actions including the inhibition of protein kinase C and cell membrane disruption. |

(±)-Palmitoylcarnitine chloride Dilution Calculator

(±)-Palmitoylcarnitine chloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2932 mL | 11.466 mL | 22.9321 mL | 45.8642 mL | 57.3302 mL |
| 5 mM | 0.4586 mL | 2.2932 mL | 4.5864 mL | 9.1728 mL | 11.466 mL |
| 10 mM | 0.2293 mL | 1.1466 mL | 2.2932 mL | 4.5864 mL | 5.733 mL |
| 50 mM | 0.0459 mL | 0.2293 mL | 0.4586 mL | 0.9173 mL | 1.1466 mL |
| 100 mM | 0.0229 mL | 0.1147 mL | 0.2293 mL | 0.4586 mL | 0.5733 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- BOP-Cl
Catalog No.:BCC2808
CAS No.:68641-49-6
- CP-945598 HCl
Catalog No.:BCC1082
CAS No.:686347-12-6
- Otenabant
Catalog No.:BCC1828
CAS No.:686344-29-6
- Procerine
Catalog No.:BCN2017
CAS No.:68622-81-1
- Xylobiose
Catalog No.:BCN8424
CAS No.:6860-47-5
- Isorhynchophylline
Catalog No.:BCN6458
CAS No.:6859-1-4
- Isorhyncophylline
Catalog No.:BCN3466
CAS No.:6859-01-4
- PX-478 2HCl
Catalog No.:BCC6502
CAS No.:685898-44-6
- Prometaphanine
Catalog No.:BCN4244
CAS No.:6858-85-1
- Moschamine
Catalog No.:BCN3900
CAS No.:68573-23-9
- Pridinol Methanesulfonate
Catalog No.:BCC3845
CAS No.:6856-31-1
- Eupatoriopicrin
Catalog No.:BCN7116
CAS No.:6856-01-5
- IWP-2
Catalog No.:BCC1665
CAS No.:686770-61-6
- BC 11-38
Catalog No.:BCC7940
CAS No.:686770-80-9
- IWP 4
Catalog No.:BCC5602
CAS No.:686772-17-8
- Qianhucoumarin G
Catalog No.:BCN3704
CAS No.:68692-61-5
- Retronecine N-oxide
Catalog No.:BCN2035
CAS No.:6870-33-3
- Jacobine
Catalog No.:BCN2087
CAS No.:6870-67-3
- 11β,17α-Dihydroxy-6α-methylpregna-1,4-diene-3,20-dione
Catalog No.:BCC8434
CAS No.:6870-94-6
- 13,18-Dehydroglaucarubinone
Catalog No.:BCN7957
CAS No.:68703-94-6
- Asimilobine
Catalog No.:BCN7076
CAS No.:6871-21-2
- Echitamine
Catalog No.:BCN4245
CAS No.:6871-44-9
- (-)-Lotusine
Catalog No.:BCN8443
CAS No.:6871-67-6
- Arteanoflavone
Catalog No.:BCN6824
CAS No.:68710-17-8
Molecular weight-dependent paracellular transport of fluorescent model compounds induced by palmitoylcarnitine chloride across the human intestinal epithelial cell line Caco-2.[Pubmed:9769019]
J Drug Target. 1998;6(1):37-43.
Long-chain acylcarnitines, such as palmitoylcarnitine chloride (PCC), are endogenous compounds which have been shown to increase intestinal transport of small hydrophilic compounds (including some pharmaceutical agents) through the paracellular pathway. However, the size range of the compounds whose absorption can be improved by PCC has not been fully investigated. In the present study, we systematically examined the effect of PCC on the transport rate of a series of hydrophilic fluorescent model compounds of varying molecular weights (0.3-71.2 kD) across cultured monolayers of the human intestinal epithelial cells Caco-2. Mucosal addition of 100 or 200 microM PCC resulted in comparable time-dependent decreases in the transepithelial electric resistance (T1/2, approximately 15 min). PCC addition induced a striking increase in the transport of sodium fluorescein (Flu-Na; 0.3 kD) and a slight or moderate increase in transports of fluorescent compounds of 0.6-11 kD. The effect of PCC on transport of compounds with molecular weights of > or = 17 kD appeared to be negligible. Examination by confocal laser scanning microscopy clearly revealed dilated paracellular spaces in Caco-2 monolayers which had been mucosally pretreated with PCC, confirming that PCC increases intestinal permeability by opening a paracellular transport pathway. Our results suggest that PCC is particularly effective in enhancing intestinal absorption of small hydrophilic compound like Flu-Na and may also have limited use in promoting the transport of compounds of < or = 10 kD.
The membrane-perturbing properties of palmitoyl-coenzyme A and palmitoylcarnitine. A comparative study.[Pubmed:7654694]
Biochemistry. 1995 Aug 22;34(33):10400-5.
Fatty acyl-coenzyme A's are temporarily converted into fatty acylcarnitines while transferred across the inner mitochondrial membrane, in their catabolic pathway. In search of an explanation for the need of this coenzyme exchange, the present work describes comparatively the abilities of both kinds of fatty acyl derivatives (represented by palmitoyl-coenzyme A and palmitoylcarnitine) in binding to and perturbing the structure of phosphatidylcholine bilayers in the form of large unilamellar vesicles. Both palmitoyl-coenzyme A and palmitoylcarnitine partition preferentially into the bilayer lipids, so that their free concentration in water is in practice negligible. However, palmitoylcarnitine is able to disrupt the membrane barrier to solutes, leading to vesicle leakage, and, at higher concentrations, it produces complete membrane solubilization, while palmitoyl-coenzyme A produces neither leakage nor solubilization. Palmitoylcarnitine has the properties of many commonly used biochemical detergents. The different behavior of both fatty acyl derivatives helps to explain the need for the transitory coenzyme A/carnitine exchange, and provides a pathogenic mechanism for some genetic defects of mitochondrial fatty acid transport. Other pathophysiological processes in which palmitoylcarnitine has been putatively involved are examined in light of the above results.
The effects of novel vasodilator long chain acyl carnitine esters in the isolated perfused heart of the rat.[Pubmed:1691947]
Br J Pharmacol. 1990 Mar;99(3):477-80.
1. The effects of palmitoyl carnitine (PC) and novel derivatives were examined on the isolated Langendorff perfused heart of the rat. 2. Bolus injections of PC (1-300 nmol) produced coronary constriction accompanied by a cumulative irreversible depression of contractility. 3. Prior storage of PC in chloroform containing 2% ethanol in heat-sealed ampoules resulted in production of the ethyl ester of the compound (PCE). This compound was isolated and also synthesized (P1E). In contrast to PC, both PCE and P1E exhibited potent vasodilator activity. 4. Increasing the fatty acid chain length from palmitoyl to stearoyl resulted in a significant reduction in coronary dilator activity of the ester compound, whereas different ester groups did not affect the vasodilator action appreciably. Complete removal of the fatty acid chain abolished all vascular effects at the doses used. 5. The vasodilatation produced by these acyl carnitine esters was comparable to that produced by several known vasodilator drugs including verapamil, cromakalim, amyl nitrate and iloprost; however, the duration of the vasodilator response was more prolonged with the carnitate derivatives.
Interactions of palmitoyl carnitine with the endothelium in rat aorta.[Pubmed:1696151]
Br J Pharmacol. 1990 Jun;100(2):241-6.
1. Palmitoyl carnitine (10-1000 microM) resembled Bay K 8644 (10-1000 nM) in that it directly contracted rat aortic rings which were partially depolarized with K+ (12 mM). However, the effects of Bay K 8644 were reduced in the presence of endothelium whereas the presence of the endothelium hardly affected the palmitoyl carnitine-induced contractions, which occurred at high concentrations (greater than 10 microM). 2. Lower concentrations of palmitoyl carnitine (0.3-30 microM; EC50 1.1 microM), but not Bay K 8644, carnitine or palmitic acid, antagonized the relaxant effects of acetylcholine in rat aorta. The antagonism was specific for endothelium-dependent relaxations, in that the relaxations to ATP and the calcium ionophore A23187 were also non-competitively antagonized, albeit at slightly higher concentrations, whereas the direct relaxant effects of sodium nitroprusside were unaffected. Palmitoyl carnitine therefore antagonizes the effects or the release of endothelial-derived relaxant factor (EDRF). The inhibitory effects were reversed on prolonged washout, indicating that the effects were not due to destruction of the endothelial cells. 3. In superfusion experiments, palmitoyl carnitine inhibited the release of EDRF from rat aorta but did not affect the responsiveness to exogenous EDRF, indicating a site of action at the endothelial cell. In superfusion experiments, palmitoyl carnitine, and lysophosphatidyl choline, caused direct relaxations of the aorta, indicating EDRF release, prior to inhibition of release evoked by receptor stimulation. These substances may modulate vascular responsiveness under certain conditions.
Modulation by palmitoylcarnitine of protein kinase C activation.[Pubmed:3479247]
Cancer Res. 1987 Dec 15;47(24 Pt 1):6537-42.
Palmitoylcarnitine, a reported protein kinase C inhibitor, enhanced the phorbol ester dependency of the enzyme, augmenting protein kinase C activity in the presence of phorbol esters such as phorbol 12,13-dibutyrate while inhibiting the basal activity measured in the presence of calcium plus phosphatidylserine. Weakly potent phorbol esters such as phorbol 12,13-diacetate and 4-O-methylphorbol 12-myristate 13-acetate were full agonists like phorbol 12,13-dibutyrate for activation of protein kinase C in the presence of palmitoylcarnitine. On the other hand, 1,2-diacylglycerols such as 1,2-diolein were only partially stimulatory. Palmitoylcarnitine did not interfere with the association of protein kinase C with phosphatidylserine, suggesting that its action was on protein kinase C activation per se rather than on priming. A long fatty acid ester, quaternary amine, and anionic charge were needed for the palmitoylcarnitine-like action. Phosphatidylcholine, which possesses these features, partially mimicked the action of palmitoylcarnitine. Palmitoylcarnitine thus appears to be a lipophilic modulator of protein kinase C rather than a simple inhibitor. The results raise the possibility that differences in response between phorbol esters and diacylglycerols may reflect differential ability to activate protein kinase C in the appropriate lipid environment rather than the existence of unique targets for one or the other compound.


