Proscillaridin ACAS# 466-06-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 466-06-8 | SDF | Download SDF |
| PubChem ID | 5284613 | Appearance | White powder |
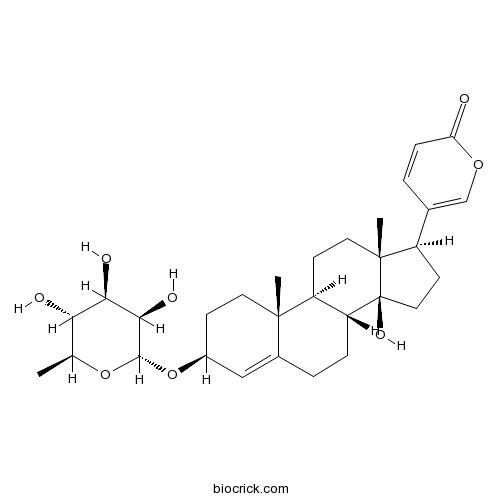
| Formula | C30H42O8 | M.Wt | 530.7 |
| Type of Compound | Isoprenoids | Storage | Desiccate at -20°C |
| Synonyms | Scillarenin 3β-rhamnoside | ||
| Solubility | Sparingly soluble in ethanol and methanol; very slightly soluble in water | ||
| Chemical Name | 5-[(3S,8R,9S,10R,13R,14S,17R)-14-hydroxy-10,13-dimethyl-3-[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy-1,2,3,6,7,8,9,11,12,15,16,17-dodecahydrocyclopenta[a]phenanthren-17-yl]pyran-2-one | ||
| SMILES | CC1C(C(C(C(O1)OC2CCC3(C4CCC5(C(CCC5(C4CCC3=C2)O)C6=COC(=O)C=C6)C)C)O)O)O | ||
| Standard InChIKey | MYEJFUXQJGHEQK-ALRJYLEOSA-N | ||
| Standard InChI | InChI=1S/C30H42O8/c1-16-24(32)25(33)26(34)27(37-16)38-19-8-11-28(2)18(14-19)5-6-22-21(28)9-12-29(3)20(10-13-30(22,29)35)17-4-7-23(31)36-15-17/h4,7,14-16,19-22,24-27,32-35H,5-6,8-13H2,1-3H3/t16-,19-,20+,21-,22+,24-,25+,26+,27-,28-,29+,30-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Proscillaridin A Dilution Calculator

Proscillaridin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8843 mL | 9.4215 mL | 18.843 mL | 37.6861 mL | 47.1076 mL |
| 5 mM | 0.3769 mL | 1.8843 mL | 3.7686 mL | 7.5372 mL | 9.4215 mL |
| 10 mM | 0.1884 mL | 0.9422 mL | 1.8843 mL | 3.7686 mL | 4.7108 mL |
| 50 mM | 0.0377 mL | 0.1884 mL | 0.3769 mL | 0.7537 mL | 0.9422 mL |
| 100 mM | 0.0188 mL | 0.0942 mL | 0.1884 mL | 0.3769 mL | 0.4711 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hedragonic acid
Catalog No.:BCN6911
CAS No.:466-02-4
- Hederagonic acid
Catalog No.:BCN5514
CAS No.:466-01-3
- Cycloartanol
Catalog No.:BCN4860
CAS No.:4657-58-3
- alpha-Spinasterol acetate
Catalog No.:BCN5510
CAS No.:4651-46-1
- Hederagenin
Catalog No.:BCN5513
CAS No.:465-99-6
- Marrubiin
Catalog No.:BCC8208
CAS No.:465-92-9
- Quinovic acid
Catalog No.:BCN5512
CAS No.:465-74-7
- Resibufogenin
Catalog No.:BCN5366
CAS No.:465-39-4
- Bufalin
Catalog No.:BCN1046
CAS No.:465-21-4
- Polyporenic acid C
Catalog No.:BCN3645
CAS No.:465-18-9
- Oleandrin
Catalog No.:BCN5511
CAS No.:465-16-7
- Neritaloside
Catalog No.:BCN5509
CAS No.:465-13-4
- Uzarigenin
Catalog No.:BCN5515
CAS No.:466-09-1
- Benzoylaconine
Catalog No.:BCN5400
CAS No.:466-24-0
- Bullatine B
Catalog No.:BCN2375
CAS No.:466-26-2
- Rauvomitin
Catalog No.:BCN3421
CAS No.:466-57-9
- Cryptomeridiol
Catalog No.:BCN5516
CAS No.:4666-84-6
- Arteminin
Catalog No.:BCN3642
CAS No.:466639-11-2
- 3-O-(2'E,4'E-Decadienoyl)ingenol
Catalog No.:BCN3768
CAS No.:466663-11-6
- 3-O-(2'E ,4'E-decadienoyl)-20-O-acetylingenol
Catalog No.:BCN1437
CAS No.:466663-12-7
- Z-Asp-OMe
Catalog No.:BCC2792
CAS No.:4668-42-2
- N-Methylcorydiniumiodide
Catalog No.:BCN7873
CAS No.:4668-64-6
- Hecogenin
Catalog No.:BCN5408
CAS No.:467-55-0
- Coronaridine
Catalog No.:BCN3762
CAS No.:467-77-6
Proscillaridin A induces apoptosis and suppresses non-small-cell lung cancer tumor growth via calcium-induced DR4 upregulation.[Pubmed:29899551]
Cell Death Dis. 2018 Jun 13;9(6):696.
Non-small-cell lung cancer (NSCLC) is the predominant histological type of lung cancer and is characterized by the highest mortality and incidence rates among these types of malignancies. Cardiac glycosides, a class of natural products, have been identified as a potential type of chemotherapeutic agent. This study aims to investigate the anti-cancer effects and the mechanisms of action of Proscillaridin A (P.A) in NSCLC cells. In vitro sodium-potassium pump (Na(+)/K(+) ATPase) enzyme assays indicated that P.A is a direct Na(+)/K(+) ATPase inhibitor. P.A showed potent cytotoxic effects in NSCLC cells at nanomolar levels. Treatment mechanism studies indicated that P.A elevated Ca(2+) levels, activated the AMPK pathway and downregulated phosphorylation of ACC and mTOR. Subsequently, P.A increased death receptor 4 (DR4) expression and downregulated NF-kappaB. Interestingly, P.A selectively suppressed EGFR activation in EGFR mutant cells but not in EGFR wild-type cells. In vivo, P.A significantly suppressed tumor growth in nude mice compared to vehicle-treated mice. Compared with the Afatinib treatment group, P.A displayed less pharmaceutical toxicity, as the body weight of mice treated with P.A did not decrease as much as those treated with Afatinib. Consistent changes in protein levels were obtained from western blotting analysis of tumors and cell lines. Immunohistochemistry analysis of the tumors from P.A-treated mice showed a significant suppression of EGFR phosphorylation (Tyr 1173) and reduction of the cell proliferation marker Ki-67. Taken together, our results suggest that P.A is a promising anti-cancer therapeutic candidate for NSCLC.
Proscillaridin A Promotes Oxidative Stress and ER Stress, Inhibits STAT3 Activation, and Induces Apoptosis in A549 Lung Adenocarcinoma Cells.[Pubmed:29576846]
Oxid Med Cell Longev. 2018 Jan 11;2018:3853409.
Cardiac glycosides are natural compounds used for the treatment of cardiovascular disorders. Although originally prescribed for cardiovascular diseases, more recently, they have been rediscovered for their potential use in the treatment of cancer. Proscillaridin A (PSD-A), a cardiac glycoside component of Urginea maritima, has been reported to exhibit anticancer activity. However, the cellular targets and anticancer mechanism of PSD-A in various cancers including lung cancer remain largely unexplored. In the present study, we found that PSD-A inhibits growth and induces apoptosis in A549 lung adenocarcinoma cells. The anticancer activity of PSD-A was found to be associated with the activation of JNK, induction of ER stress, mitochondrial dysfunction, and inhibition of STAT3 activation. PSD-A induces oxidative stress as evidenced from ROS generation, GSH depletion, and decreased activity of TrxR1. PSD-A-mediated ER stress was verified by increased phosphorylation of eIF2alpha and expression of its downstream effector proteins ATF4, CHOP, and caspases-4. PSD-A triggered apoptosis by inducing JNK (1/2) activation, increasing bax/bcl-2 ratio, dissipating mitochondrial membrane potential, and inducing cleavage of caspases and PARP. Further study revealed that PSD-A inhibits both constitutive and inducible STAT3 activations and decreases STAT3 DNA-binding activity. Moreover, PSD-A-mediated inhibition of STAT3 activation was found to be associated with increased SHP-1 expression, decreased phosphorylation of Src, and binding of PSD-A with STAT3 SH2 domain. Finally, STAT3 knockdown by shRNA inhibited growth and enhanced apoptotic efficacy of PSD-A. Taken together, the data suggest that PSD-A could be developed into a potential therapeutic agent against lung adenocarcinoma.


