MarrubiinCAS# 465-92-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 465-92-9 | SDF | Download SDF |
| PubChem ID | 73401 | Appearance | White powder |
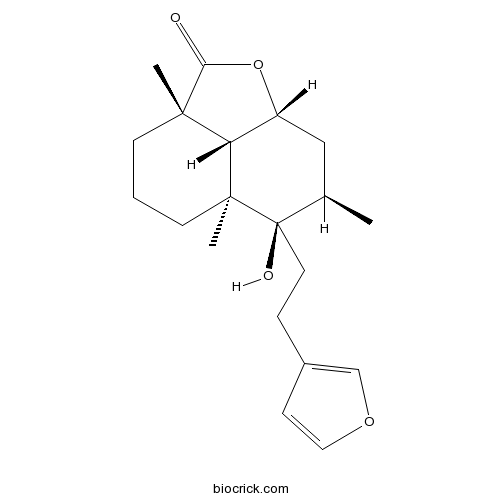
| Formula | C20H28O4 | M.Wt | 332.4 |
| Type of Compound | Isoprenoids | Storage | Desiccate at -20°C |
| Synonyms | Marrubin | ||
| Solubility | Soluble in acetonitrile, diethyl ether and ethan | ||
| Chemical Name | None | ||
| SMILES | CC1CC2C3C(CCCC3(C1(CCC4=COC=C4)O)C)(C(=O)O2)C | ||
| Standard InChIKey | HQLLRHCTVDVUJB-OBHOOXMTSA-N | ||
| Standard InChI | InChI=1S/C20H28O4/c1-13-11-15-16-18(2,17(21)24-15)7-4-8-19(16,3)20(13,22)9-5-14-6-10-23-12-14/h6,10,12-13,15-16,22H,4-5,7-9,11H2,1-3H3/t13-,15-,16+,18+,19+,20-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Marrubiin Dilution Calculator

Marrubiin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0084 mL | 15.0421 mL | 30.0842 mL | 60.1685 mL | 75.2106 mL |
| 5 mM | 0.6017 mL | 3.0084 mL | 6.0168 mL | 12.0337 mL | 15.0421 mL |
| 10 mM | 0.3008 mL | 1.5042 mL | 3.0084 mL | 6.0168 mL | 7.5211 mL |
| 50 mM | 0.0602 mL | 0.3008 mL | 0.6017 mL | 1.2034 mL | 1.5042 mL |
| 100 mM | 0.0301 mL | 0.1504 mL | 0.3008 mL | 0.6017 mL | 0.7521 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Quinovic acid
Catalog No.:BCN5512
CAS No.:465-74-7
- Resibufogenin
Catalog No.:BCN5366
CAS No.:465-39-4
- Bufalin
Catalog No.:BCN1046
CAS No.:465-21-4
- Polyporenic acid C
Catalog No.:BCN3645
CAS No.:465-18-9
- Oleandrin
Catalog No.:BCN5511
CAS No.:465-16-7
- Neritaloside
Catalog No.:BCN5509
CAS No.:465-13-4
- Gamabufotalin
Catalog No.:BCN2358
CAS No.:465-11-2
- Germanicol
Catalog No.:BCN7507
CAS No.:465-02-1
- Arjunolic acid
Catalog No.:BCN5508
CAS No.:465-00-9
- Pseudotaraxasterol
Catalog No.:BCN5507
CAS No.:464-98-2
- Asiatic acid
Catalog No.:BCN5506
CAS No.:464-92-6
- Conquinamine
Catalog No.:BCN6622
CAS No.:464-86-8
- Hederagenin
Catalog No.:BCN5513
CAS No.:465-99-6
- alpha-Spinasterol acetate
Catalog No.:BCN5510
CAS No.:4651-46-1
- Cycloartanol
Catalog No.:BCN4860
CAS No.:4657-58-3
- Hederagonic acid
Catalog No.:BCN5514
CAS No.:466-01-3
- Hedragonic acid
Catalog No.:BCN6911
CAS No.:466-02-4
- Proscillaridin A
Catalog No.:BCC8239
CAS No.:466-06-8
- Uzarigenin
Catalog No.:BCN5515
CAS No.:466-09-1
- Benzoylaconine
Catalog No.:BCN5400
CAS No.:466-24-0
- Bullatine B
Catalog No.:BCN2375
CAS No.:466-26-2
- Rauvomitin
Catalog No.:BCN3421
CAS No.:466-57-9
- Cryptomeridiol
Catalog No.:BCN5516
CAS No.:4666-84-6
- Arteminin
Catalog No.:BCN3642
CAS No.:466639-11-2
Marrubiin-loaded solid lipid nanoparticles' impact on TNF-alpha treated umbilical vein endothelial cells: A study for cardioprotective effect.[Pubmed:29413609]
Colloids Surf B Biointerfaces. 2018 Apr 1;164:299-307.
Oxidative stress possesses a key role in the onset and development of cardiovascular diseases (CVDs), thus it can be an efficient target to tackle such ailment. Marrubiin, a bioactive diterpene, is a potent antioxidant against oxidative stress. Herein, we aimed to formulate Marrubiin loaded solid lipid nanoparticles (SLNs) to improve its pharmacokinetics and bioavailability and also to investigate free drug and formulation's protective impact against intracellular reactive oxygen species (ROS) generation in HUVECs. Marrubiin-SLNs were formulated using hot homogenization/solidification method and then were subjected to physicochemical characterizations, i.e. size, zeta potential, morphology, polydispersity index (PDI), encapsulation efficiency (% EE), drug loading/content and physical stability assessments. MTT assay was performed to study the cytotoxicity of the intact and SLN incorporated Marrubiin on HUVECs. Further, the antioxidant property of Marrubiin and formulations was evaluated using DPPH radical scavenging assay and their protective effect against TNF-alpha induced oxidative stress was assessed by the means of intracellular ROS assessment, and also apoptosis/necrosis, cell cycle, and DNA fragmentation assays. Electron microscopy analysis showed spherical monodispersed SLNs with the size less than 100nm, particle/zeta size analyses also approved the size of particles with a zeta potential of -1.28+/-0.17mV. Results also showed high EE (98%), drug loading (31.74mg/g) with 3.15% drug content. In vitro release studies revealed about 90% of Marrubiin cumulative release during 24h. The stability of Marrubiin-SLNs in terms of size, zeta potential, polydispersity index, EE and drug leakage was approved. Marrubiin antioxidant stability after formulation was approved by DPPH analysis. MTT cell survival assay showed no significant cytotoxicity after 24h and 48h. Intracellular ROS detection assay revealed that Marrubiin and Marrubiin-SLNs, play protective effect against TNF-alpha induced oxidative stress in HUVECs which was further approved by apoptosis assessment. Conclusively, based on our findings, Marrubiin nanoparticles are proposed as a preventive/therapeutic remedy against disorders elicited by increased levels of intracellular ROS in CVDs.
Total Syntheses of (+)-Marrubiin and (-)-Marrulibacetal.[Pubmed:27341320]
Org Lett. 2016 Jul 15;18(14):3430-3.
A stereoselective total synthesis of (+)-Marrubiin has been accomplished starting from a chiral building block via the CyNH2-promoted Pauson-Khand reaction (PKR) followed by oxidative cleavage of the resultant cyclopentenone ring. Two successive oxidations and internal transacetalization culminated in the total synthesis of the antispasmodic labdane diterpenoid (-)-marrulibacetal.
Protective effects of marrubiin improve endometriosis through suppression of the expression of RANTES.[Pubmed:28713901]
Mol Med Rep. 2017 Sep;16(3):3339-3344.
Marrubiin can improve blood and lymph microcirculation disturbance, and has pharmacological effects in myocardial protection, antiinflammation and antioxidation. The aim of the present study was to evaluate the protective effects of Marrubiin on endometriosis through suppression of the expression of regulated on activation, normal T cell expressed and secreted (RANTES). Endometriotic cells were implanted into the peritoneal cavity of mice, and these mice were injected estradiol benzoate (30 microg/kg) once each day for 14 days. The mice with endometriosis were then treated with 12, 25 or 50 mg/kg Marrubiin. Reverse transcriptionquantitative polymerase chain reaction was used to assess the mRNA expression of RANTES, and western blot analysis was used to analyze the protein expression of RANTES, TNFalpha and PGE2. Inflammation factors were measured by ELISA. Treatment with Marrubiin effectively improved lesion regression and inhibited toxicity in the mouse model of endometriosis. Marrubiin significantly inhibited endometrial lesions and monocyte chemotaxis in the mice with endometriosis, and reduced U937 cell migration. Calcium mobilization, levels of tumor necrosis factoralpha and the secretion of RANTES were effectively suppressed by Marrubiin treatment. The calcium levels were effectively induced, whereas the protein expression of prostaglandin E2 (PGE2) and formation of thromboxane B2 (TXB2) were effectively inhibited by Marrubiin treatment. These findings indicated that the protective effect of Marrubiin improved endometriosis in the mice through the suppression of inflammation and downregulation of the expression of RANTES, followed by mediation of the levels of calcium, PGE2 and TXB2.


