GamabufotalinCAS# 465-11-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 465-11-2 | SDF | Download SDF |
| PubChem ID | 259803 | Appearance | Powder |
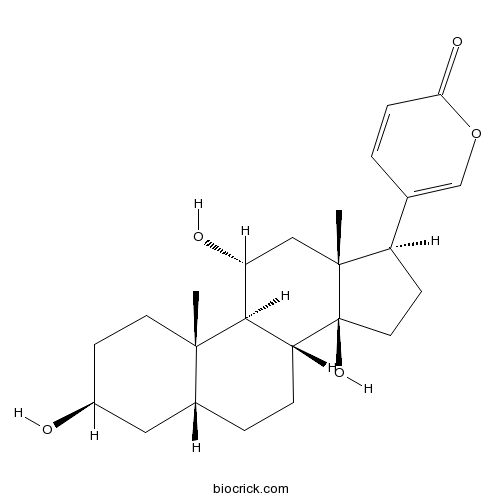
| Formula | C24H34O5 | M.Wt | 402.52 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Synonyms | Gamabufagin | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-[(3S,5R,8R,9S,10S,11R,13R,14S,17R)-3,11,14-trihydroxy-10,13-dimethyl-1,2,3,4,5,6,7,8,9,11,12,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]pyran-2-one | ||
| SMILES | CC12CCC(CC1CCC3C2C(CC4(C3(CCC4C5=COC(=O)C=C5)O)C)O)O | ||
| Standard InChIKey | FMTLOAVOGWSPEF-KJRPADTMSA-N | ||
| Standard InChI | InChI=1S/C24H34O5/c1-22-9-7-16(25)11-15(22)4-5-18-21(22)19(26)12-23(2)17(8-10-24(18,23)28)14-3-6-20(27)29-13-14/h3,6,13,15-19,21,25-26,28H,4-5,7-12H2,1-2H3/t15-,16+,17-,18-,19-,21-,22+,23-,24+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Gamabufotalin has been used for treatment of COX-2-mediated diseases and cancer therapy, it triggers c-Myc degradation via induction of WWP2 in multiple myeloma(MM) cells. Gamabufotalin strongly inhibit cancer cell growth and inflammatory response, it inhibits angiogenesis by inhibiting the activation of VEGFR-2 signaling pathways and could be a potential candidate in angiogenesis-related disease therapy. |
| Targets | COX | p65 | NF-kB | IkB | VEGFR | c-Myc | JNK | IKK |
| In vitro | Gamabufotalin, a major derivative of bufadienolide, inhibits VEGF-induced angiogenesis by suppressing VEGFR-2 signaling pathway.[Pubmed: 26657289 ]Oncotarget. 2016 Jan 19;7(3):3533-47.Gamabufotalin (CS-6), a main active compound isolated from Chinese medicine Chansu, has been shown to strongly inhibit cancer cell growth and inflammatory response. However, its effects on angiogenesis have not been known yet. Gamabufotalin triggers c-Myc degradation via induction of WWP2 in multiple myeloma cells.[Pubmed: 26894970 ]Oncotarget. 2016 Mar 29;7(13):15725-37.Deciding appropriate therapy for multiple myeloma (MM) is challenging because of the occurrence of multiple chromosomal changes and the fatal nature of the disease. |
| Kinase Assay | Gamabufotalin, a bufadienolide compound from toad venom, suppresses COX-2 expression through targeting IKKβ/NF-κB signaling pathway in lung cancer cells.[Pubmed: 25175164]Mol Cancer. 2014 Aug 31;13:203.Gamabufotalin (CS-6), a major bufadienolide of Chansu, has been used for cancer therapy due to its desirable metabolic stability and less adverse effect. However, the underlying mechanism of Gamabufotalin involved in anti-tumor activity remains poorly understood. |

Gamabufotalin Dilution Calculator

Gamabufotalin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4843 mL | 12.4217 mL | 24.8435 mL | 49.687 mL | 62.1087 mL |
| 5 mM | 0.4969 mL | 2.4843 mL | 4.9687 mL | 9.9374 mL | 12.4217 mL |
| 10 mM | 0.2484 mL | 1.2422 mL | 2.4843 mL | 4.9687 mL | 6.2109 mL |
| 50 mM | 0.0497 mL | 0.2484 mL | 0.4969 mL | 0.9937 mL | 1.2422 mL |
| 100 mM | 0.0248 mL | 0.1242 mL | 0.2484 mL | 0.4969 mL | 0.6211 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Gamabufotalin (Gamabufagin), a major bufadienolide of Chansu, has been used for cancer therapy due to its desirable metabolic stability and less adverse effect. IC50 value: Target: in vitro: Gamabufotalin (CS-6) strongly suppressed COX-2 expression by inhibiting the phosphorylation of IKKβ via targeting the ATP-binding site, thereby abrogating NF-κB binding and p300 recruitment to COX-2 promoter. In addition, CS-6 induced apoptosis by activating the cytochrome c and caspase-dependent apoptotic pathway [1]. Gamabufotalin significantly potentiated human breast cancer cells with different status of ER-alpha to apoptosis induction of TRAIL, as evidenced by enhanced Annexin V/FITC positive cells (apoptotic cells), cytoplasmic histone-associated-DNA-fragments, membrane permeability transition (MPT), caspases activation and PARP cleavage [2]. in vivo: CS-6 markedly down-regulated the protein levels of COX-2 and phosphorylated p65 NF-κB in tumor tissues of the xenograft mice, and inhibited tumor weight and size [1].
References:
[1]. Yu Z, et al. Gamabufotalin, a bufadienolide compound from toad venom, suppresses COX-2 expression through targeting IKKβ/NF-κB signaling pathway in lung cancer cells. Mol Cancer. 2014 Aug 31;13:203.
[2]. Dong Y, et al. Bufadienolide compounds sensitize human breast cancer cells to TRAIL-induced apoptosis via inhibition of STAT3/Mcl-1 pathway. Apoptosis. 2011 Apr;16(4):394-403.
- Germanicol
Catalog No.:BCN7507
CAS No.:465-02-1
- Arjunolic acid
Catalog No.:BCN5508
CAS No.:465-00-9
- Pseudotaraxasterol
Catalog No.:BCN5507
CAS No.:464-98-2
- Asiatic acid
Catalog No.:BCN5506
CAS No.:464-92-6
- Conquinamine
Catalog No.:BCN6622
CAS No.:464-86-8
- Quinamine
Catalog No.:BCN6590
CAS No.:464-85-7
- Arenobufagin
Catalog No.:BCN5401
CAS No.:464-74-4
- Benzopinacol
Catalog No.:BCC8860
CAS No.:464-72-2
- (+)-Camphor
Catalog No.:BCN7161
CAS No.:464-49-3
- (-)-Camphor
Catalog No.:BCN7160
CAS No.:464-48-2
- (-)-Borneol
Catalog No.:BCC8897
CAS No.:464-45-9
- (+)-Borneol
Catalog No.:BCC8376
CAS No.:464-43-7
- Neritaloside
Catalog No.:BCN5509
CAS No.:465-13-4
- Oleandrin
Catalog No.:BCN5511
CAS No.:465-16-7
- Polyporenic acid C
Catalog No.:BCN3645
CAS No.:465-18-9
- Bufalin
Catalog No.:BCN1046
CAS No.:465-21-4
- Resibufogenin
Catalog No.:BCN5366
CAS No.:465-39-4
- Quinovic acid
Catalog No.:BCN5512
CAS No.:465-74-7
- Marrubiin
Catalog No.:BCC8208
CAS No.:465-92-9
- Hederagenin
Catalog No.:BCN5513
CAS No.:465-99-6
- alpha-Spinasterol acetate
Catalog No.:BCN5510
CAS No.:4651-46-1
- Cycloartanol
Catalog No.:BCN4860
CAS No.:4657-58-3
- Hederagonic acid
Catalog No.:BCN5514
CAS No.:466-01-3
- Hedragonic acid
Catalog No.:BCN6911
CAS No.:466-02-4
Gamabufotalin triggers c-Myc degradation via induction of WWP2 in multiple myeloma cells.[Pubmed:26894970]
Oncotarget. 2016 Mar 29;7(13):15725-37.
Deciding appropriate therapy for multiple myeloma (MM) is challenging because of the occurrence of multiple chromosomal changes and the fatal nature of the disease. In the current study, Gamabufotalin (GBT) was isolated from toad venom, and its tumor-specific cytotoxicity was investigated in human MM cells. We found GBT inhibited cell growth and induced apoptosis with the IC50 values <50 nM. Mechanistic studies using functional approaches identified GBT as an inhibitor of c-Myc. Further analysis showed that GBT especially evoked the ubiquitination and degradation of c-Myc protein, thereby globally repressing the expression of c-Myc target genes. GBT treatment inhibited ERK and AKT signals, while stimulating the activation of JNK cascade. An E3 ubiquitin-protein ligase, WWP2, was upregulated following JNK activation and played an important role in c-Myc ubiquitination and degradation through direct protein-protein interaction. The antitumor effect of GBT was validated in a xenograft mouse model and the suppression of MM-induced osteolysis was verified in a SCID-hu model in vivo. Taken together, our study identified the potential of GBT as a promising therapeutic agent in the treatment of MM.
Gamabufotalin, a bufadienolide compound from toad venom, suppresses COX-2 expression through targeting IKKbeta/NF-kappaB signaling pathway in lung cancer cells.[Pubmed:25175164]
Mol Cancer. 2014 Aug 31;13:203.
BACKGROUND: Gamabufotalin (CS-6), a major bufadienolide of Chansu, has been used for cancer therapy due to its desirable metabolic stability and less adverse effect. However, the underlying mechanism of CS-6 involved in anti-tumor activity remains poorly understood. METHODS: The biological functions of Gamabufotalin (CS-6) were investigated by migration, colony formation and apoptosis assays in NSCLC cells. The nuclear localization and interaction between transcriptional co-activator p300 and NF-kappaB p50/p65 and their binding to COX-2 promoter were analyzed after treatment with CS-6. Molecular docking study was used to simulate the interaction of CS-6 with IKKbeta. The in vivo anti-tumor efficacy of CS-6 was also analyzed in xenografts nude mice. Western blot was used to detect the protein expression level. RESULTS: Gamabufotalin (CS-6) strongly suppressed COX-2 expression by inhibiting the phosphorylation of IKKbeta via targeting the ATP-binding site, thereby abrogating NF-kappaB binding and p300 recruitment to COX-2 promoter. In addition, CS-6 induced apoptosis by activating the cytochrome c and caspase-dependent apoptotic pathway. Moreover, CS-6 markedly down-regulated the protein levels of COX-2 and phosphorylated p65 NF-kappaB in tumor tissues of the xenograft mice, and inhibited tumor weight and size. CONCLUSIONS: Our study provides pharmacological evidence that CS-6 exhibits potential use in the treatment of COX-2-mediated diseases such as lung cancer.
Gamabufotalin, a major derivative of bufadienolide, inhibits VEGF-induced angiogenesis by suppressing VEGFR-2 signaling pathway.[Pubmed:26657289]
Oncotarget. 2016 Jan 19;7(3):3533-47.
Gamabufotalin (CS-6), a main active compound isolated from Chinese medicine Chansu, has been shown to strongly inhibit cancer cell growth and inflammatory response. However, its effects on angiogenesis have not been known yet. Here, we sought to determine the biological effects of CS-6 on signaling mechanisms during angiogenesis. Our present results fully demonstrate that CS-6 could significantly inhibit VEGF triggered HUVECs proliferation, migration, invasion and tubulogenesis in vitro and blocked vascularization in Matrigel plugs impregnated in C57/BL6 mice as well as reduced vessel density in human lung tumor xenograft implanted in nude mice. Computer simulations revealed that CS-6 interacted with the ATP-binding sites of VEGFR-2 using molecular docking. Furthermore, western blot analysis indicated that CS-6 inhibited VEGF-induced phosphorylation of VEGFR-2 kinase and suppressed the activity of VEGFR-2-mediated signaling cascades. Therefore, our studies demonstrated that CS-6 inhibited angiogenesis by inhibiting the activation of VEGFR-2 signaling pathways and CS-6 could be a potential candidate in angiogenesis-related disease therapy.


