QuinamineCAS# 464-85-7 |
- Conquinamine
Catalog No.:BCN6622
CAS No.:464-86-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 464-85-7 | SDF | Download SDF |
| PubChem ID | 94145 | Appearance | Powder |
| Formula | C19H24N2O2 | M.Wt | 312.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
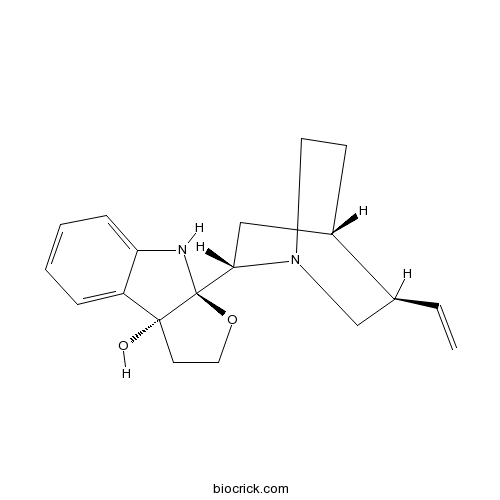
| Chemical Name | (3aS,8bR)-3a-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-2,4-dihydro-1H-furo[2,3-b]indol-8b-ol | ||
| SMILES | C=CC1CN2CCC1CC2C34C(CCO3)(C5=CC=CC=C5N4)O | ||
| Standard InChIKey | ALNKTVLUDWIWIH-HLQCWHFUSA-N | ||
| Standard InChI | InChI=1S/C19H24N2O2/c1-2-13-12-21-9-7-14(13)11-17(21)19-18(22,8-10-23-19)15-5-3-4-6-16(15)20-19/h2-6,13-14,17,20,22H,1,7-12H2/t13-,14-,17-,18+,19-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| Structure Identification | Journal of Natural Products,2004,67(10):1667-71.Five new alkaloids from the leaves of Remijia peruviana[Reference: WebLink]
|

Quinamine Dilution Calculator

Quinamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.201 mL | 16.0051 mL | 32.0102 mL | 64.0205 mL | 80.0256 mL |
| 5 mM | 0.6402 mL | 3.201 mL | 6.402 mL | 12.8041 mL | 16.0051 mL |
| 10 mM | 0.3201 mL | 1.6005 mL | 3.201 mL | 6.402 mL | 8.0026 mL |
| 50 mM | 0.064 mL | 0.3201 mL | 0.6402 mL | 1.2804 mL | 1.6005 mL |
| 100 mM | 0.032 mL | 0.1601 mL | 0.3201 mL | 0.6402 mL | 0.8003 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Arenobufagin
Catalog No.:BCN5401
CAS No.:464-74-4
- Benzopinacol
Catalog No.:BCC8860
CAS No.:464-72-2
- (+)-Camphor
Catalog No.:BCN7161
CAS No.:464-49-3
- (-)-Camphor
Catalog No.:BCN7160
CAS No.:464-48-2
- (-)-Borneol
Catalog No.:BCC8897
CAS No.:464-45-9
- (+)-Borneol
Catalog No.:BCC8376
CAS No.:464-43-7
- Bay 55-9837
Catalog No.:BCC5932
CAS No.:463930-25-8
- alpha-Linolenic acid
Catalog No.:BCN8319
CAS No.:463-40-1
- Gnemonol B
Catalog No.:BCN3399
CAS No.:462636-74-4
- Lactulose
Catalog No.:BCC4669
CAS No.:4618-18-2
- 4beta,12-dihydroxyguaian-6,10-diene
Catalog No.:BCN7829
CAS No.:461644-90-6
- Dapagliflozin
Catalog No.:BCC2552
CAS No.:461432-26-8
- Conquinamine
Catalog No.:BCN6622
CAS No.:464-86-8
- Asiatic acid
Catalog No.:BCN5506
CAS No.:464-92-6
- Pseudotaraxasterol
Catalog No.:BCN5507
CAS No.:464-98-2
- Arjunolic acid
Catalog No.:BCN5508
CAS No.:465-00-9
- Germanicol
Catalog No.:BCN7507
CAS No.:465-02-1
- Gamabufotalin
Catalog No.:BCN2358
CAS No.:465-11-2
- Neritaloside
Catalog No.:BCN5509
CAS No.:465-13-4
- Oleandrin
Catalog No.:BCN5511
CAS No.:465-16-7
- Polyporenic acid C
Catalog No.:BCN3645
CAS No.:465-18-9
- Bufalin
Catalog No.:BCN1046
CAS No.:465-21-4
- Resibufogenin
Catalog No.:BCN5366
CAS No.:465-39-4
- Quinovic acid
Catalog No.:BCN5512
CAS No.:465-74-7
Palladium-catalyzed intramolecular transfer hydrogenation & cycloaddition of p-quinamine-tethered alkylidenecyclopropanes to synthesize perhydroindole scaffolds.[Pubmed:30474659]
Chem Commun (Camb). 2018 Dec 13;54(100):14085-14088.
A palladium(0)-catalyzed intramolecular transfer hydrogenation and cycloaddition of p-Quinamine-tethered alkylidenecyclopropanes (ACPs) to synthesize perhydroindole scaffolds has been reported in this communication. Mechanistic investigations on the basis of deuterium labeling experiments suggest that the reaction proceeded through an oxidative addition of Pd(0) into the distal bond of the ACP moiety to afford a trimethylenemethane (TMM)-Pd intermediate followed by transfer hydrogenation using alcohol.
(3 + 2)-Annulation of p-Quinamine and Aryne: A Strategy To Construct the Multisubstituted Hydrocarbazoles.[Pubmed:28657328]
Org Lett. 2017 Jul 7;19(13):3600-3603.
A strategy for the synthesis of multisubstituted hydrocarbazoles has been developed through (3 + 2)-annulation of p-Quinamines and arynes. In this way, new analogs of hydrocarbazoles with quaternary carbon center can be synthesized in satisfactory yield under mild conditions. Furthermore, this (3 + 2)-annulation can be easily scaled-up, and the products can be modified through simple transformation.
Synthesis of 4-aminotropones from [(sulfinyl or sulfonyl)methyl]-substituted p-quinamines.[Pubmed:17918757]
Chemistry. 2008;14(2):621-36.
An efficient synthesis of 4-aminotropones has been achieved in excellent yields by simple treatment of 4-amino-4-[(p-tolylsulfinyl)methyl]-2,5-cyclohexadienones (p-Quinamines) with NaH. The method allowed regiocontrolled access to 3-methyl, 5-methyl- and 3,5-dimethyl-substituted derivatives starting from p-Quinamines with adequate substituents at the cyclohexadienone moiety and/or at the carbon linked to the sulfur function. The p-Quinamines in turn were easily accessible from N-Boc p-anisidines (Boc=tert-butoxycarbonyl) by electrochemical oxidation in MeOH to quinone imine monoketals, followed by addition of a alpha-lithium sulfinyl carbanion to the imino group, and ketal hydrolysis. Oxidation of the sulfoxide gave the sulfonyl-substituted p-Quinamines that, upon basic treatment, behave similarly. The p-Quinamine 55 and bis-p-Quinamine 56, resulting in the addition of the anion derived from dimethyl sulfone to the p-quinonimine ketal 14, also gave the 4-aminotropone. The mechanism involves the initial formation of a alpha-sulfonyl carbanion, which intramolecularly attacks the cyclohexadienone giving a norcaradiene-like enolate intermediate, the evolution of which through a ring-expansion process, pushes off a methyl sulfinate anion or SO2. This efficient process fulfils the criteria of atom economy. The introduction of a proline substituent in the nitrogen of the starting p-Quinamine allowed the synthesis of an enantiopure 4-aminotropone, the asymmetric Diels-Alder reactions of which with maleimide occurred in a highly endo and pi-facial diastereoselective manner.
Ring expansion of sulfur substituted p-quinamines: regiospecific synthesis of 4-aminotropones.[Pubmed:15719098]
Chem Commun (Camb). 2005 Feb 28;(8):1007-9.
Synthesis of 4-aminotropones through a cyclization-ring expansion process occurs in a single step and with excellent yields from 4-amino-2,5-cyclohexadienones (p-Quinamines) bearing a 4-sulfinyl or sulfonyl methyl group.
Five new alkaloids from the leaves of Remijia peruviana.[Pubmed:15497937]
J Nat Prod. 2004 Oct;67(10):1667-71.
Three new indolylquinuclidine-type alkaloids, remijinine (1), epiremijinine (2), and 5-acetyl-apocinchonamine (3), and two new cinchonine-derived alkaloids, N-acetyl-deoxycinchonicinol (4) and N-acetyl-cinchonicinol (5), as well as the known alkaloids Quinamine, conQuinamine, cinchonine, and quinidine were isolated from the leaves of Remijia peruviana. The structures of the new alkaloids were elucidated on the basis of spectroscopic analysis, including homonuclear and heteronuclear correlation NMR experiments (COSY, ROESY, HMQC, and HMBC). The relative configuration at C-7 for remijinine (1) and, in consequence, for epiremijinine (2) was established by X-ray crystal structure analysis of the former.
Alkaloid Production by Transformed Root Cultures of Cinchona ledgeriana.[Pubmed:17262435]
Planta Med. 1989 Aug;55(4):354-7.
Transformed root cultures of CINCHONA LEDGERIANA have been generated by infecting shoots cultured IN VITRO with AGROBACTERIUM RHIZOGENES LBA9402. These root cultures grow axenically in the absence of antibiotics or exogenous plant growth regulators in media containing Gamborg B5 salts at half or full strength with 3% (w/v) sucrose as the carbon source. They show a 6- to 8-fold increase in fresh weight in 28 days. Transformation has been confirmed by Southern blotting using [ (32)P]-labelled fragments from both the T (L)- and T (R)-DNA of the Ri plasmid. The cultures are shown to synthesise a range of quinoline alkaloids, of which quinine, cinchonidine, and quinidine are the major components. The level of these alkaloids changes with the age of the cultures, reaching a maximum at about 50 microg/g fresh weight after 45 days. In addition, the roots contain Quinamine and a number of other, unidentified, indole alkaloids, one of which is the major alkaloid present. Only about 1% of the total alkaloid present is released to the medium.


