BenzopinacolCAS# 464-72-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 464-72-2 | SDF | Download SDF |
| PubChem ID | 94766 | Appearance | Powder |
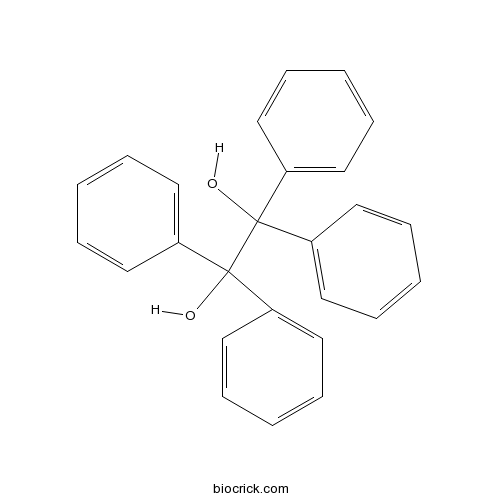
| Formula | C26H22O2 | M.Wt | 366.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,1,2,2-tetraphenylethane-1,2-diol | ||
| SMILES | C1=CC=C(C=C1)C(C2=CC=CC=C2)(C(C3=CC=CC=C3)(C4=CC=CC=C4)O)O | ||
| Standard InChIKey | MFEWNFVBWPABCX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H22O2/c27-25(21-13-5-1-6-14-21,22-15-7-2-8-16-22)26(28,23-17-9-3-10-18-23)24-19-11-4-12-20-24/h1-20,27-28H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Benzopinacol Dilution Calculator

Benzopinacol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7285 mL | 13.6426 mL | 27.2851 mL | 54.5703 mL | 68.2128 mL |
| 5 mM | 0.5457 mL | 2.7285 mL | 5.457 mL | 10.9141 mL | 13.6426 mL |
| 10 mM | 0.2729 mL | 1.3643 mL | 2.7285 mL | 5.457 mL | 6.8213 mL |
| 50 mM | 0.0546 mL | 0.2729 mL | 0.5457 mL | 1.0914 mL | 1.3643 mL |
| 100 mM | 0.0273 mL | 0.1364 mL | 0.2729 mL | 0.5457 mL | 0.6821 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-Camphor
Catalog No.:BCN7161
CAS No.:464-49-3
- (-)-Camphor
Catalog No.:BCN7160
CAS No.:464-48-2
- (-)-Borneol
Catalog No.:BCC8897
CAS No.:464-45-9
- (+)-Borneol
Catalog No.:BCC8376
CAS No.:464-43-7
- Bay 55-9837
Catalog No.:BCC5932
CAS No.:463930-25-8
- alpha-Linolenic acid
Catalog No.:BCN8319
CAS No.:463-40-1
- Gnemonol B
Catalog No.:BCN3399
CAS No.:462636-74-4
- Lactulose
Catalog No.:BCC4669
CAS No.:4618-18-2
- 4beta,12-dihydroxyguaian-6,10-diene
Catalog No.:BCN7829
CAS No.:461644-90-6
- Dapagliflozin
Catalog No.:BCC2552
CAS No.:461432-26-8
- Ko 143
Catalog No.:BCC1684
CAS No.:461054-93-3
- Eact
Catalog No.:BCC6313
CAS No.:461000-66-8
- Arenobufagin
Catalog No.:BCN5401
CAS No.:464-74-4
- Quinamine
Catalog No.:BCN6590
CAS No.:464-85-7
- Conquinamine
Catalog No.:BCN6622
CAS No.:464-86-8
- Asiatic acid
Catalog No.:BCN5506
CAS No.:464-92-6
- Pseudotaraxasterol
Catalog No.:BCN5507
CAS No.:464-98-2
- Arjunolic acid
Catalog No.:BCN5508
CAS No.:465-00-9
- Germanicol
Catalog No.:BCN7507
CAS No.:465-02-1
- Gamabufotalin
Catalog No.:BCN2358
CAS No.:465-11-2
- Neritaloside
Catalog No.:BCN5509
CAS No.:465-13-4
- Oleandrin
Catalog No.:BCN5511
CAS No.:465-16-7
- Polyporenic acid C
Catalog No.:BCN3645
CAS No.:465-18-9
- Bufalin
Catalog No.:BCN1046
CAS No.:465-21-4
Synthesis, spectroscopy and photochemistry of novel branched fluorescent nitro-stilbene derivatives with benzopheonone groups.[Pubmed:20177746]
J Fluoresc. 2010 May;20(3):703-12.
In this article, we presented novel nitro-stilbene derivatives with one or two benzophenone groups as photoinitiators via multi-steps synthesis. The ultraviolet/visible spectroscopy and the emission spectroscopy of the compounds were determined in various solvents. The results showed that the ultraviolet/visible absorption spectroscopy of the derivatives with benzophenone moiety displayed overlap effects of nitro-stilbene and benzophenone parts. In non-polar solvents, the derivatives exhibited strong emission, while they displayed weak emission in modest and strong polar solvents. Dyes-linked benzopheonone groups displayed stronger fluorescence emission than simple chromophore parent molecules. Visible-light photoinitiating effects of the derivatives were investigated extensively. Methyl methacrylate could be photoinitiated efficiently by the derivatives with benzophenone moieties at very low concentration, even at 1 x 10(-5) mol/L. While the photopolymerization efficiency of styrene initiated by the derivatives was lower than that of methyl methacrylate. Our results showed that the dye-linked photoinitators had more efficient photoinitiating than the simple mixture of dye and photoinitator. Furthermore, the derivative with two benzophenone groups displayed more excellent photoiniatiating effects than the derivative with one benzophenone group. Thermodynamics driving for the occurrence of visible-light photoinduced intramolecular electron transfer from chromophore part to benzophenone part was evaluated. Benzopinacol moiety produced in photoreaction was confirmed by nuclear magnetic resonant spectroscopy. Thermal stability of the derivatives was analyzed.


