EactActivator of Ca2+-activated Cl- channel transmembrane protein 16A (TMEM16A) CAS# 461000-66-8 |
- L 006235
Catalog No.:BCC2361
CAS No.:294623-49-7
- E-64
Catalog No.:BCC1222
CAS No.:66701-25-5
- E-64-c
Catalog No.:BCC3588
CAS No.:76684-89-4
- Cysteine Protease inhibitor
Catalog No.:BCC5301
CAS No.:921625-62-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 461000-66-8 | SDF | Download SDF |
| PubChem ID | 3173542 | Appearance | Powder |
| Formula | C22H24N2O5S | M.Wt | 428.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 10 mM in ethanol | ||
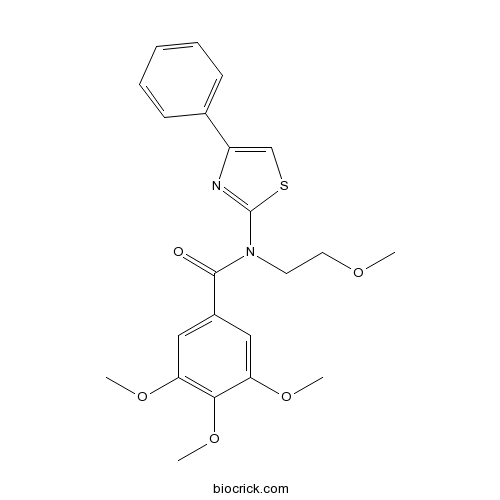
| Chemical Name | 3,4,5-trimethoxy-N-(2-methoxyethyl)-N-(4-phenyl-1,3-thiazol-2-yl)benzamide | ||
| SMILES | COCCN(C1=NC(=CS1)C2=CC=CC=C2)C(=O)C3=CC(=C(C(=C3)OC)OC)OC | ||
| Standard InChIKey | ZUXNHFFVQWADJL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H24N2O5S/c1-26-11-10-24(22-23-17(14-30-22)15-8-6-5-7-9-15)21(25)16-12-18(27-2)20(29-4)19(13-16)28-3/h5-9,12-14H,10-11H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | TMEM16A (ANO1) calcium-activated chloride channel (CaCC) activator (EC50 = 3 μM). Ca2+-independent. Displays no effect on CFTR Cl- or ENaC Na+ conductance. Stimulates submucosal gland secretion in human bronchi. |

Eact Dilution Calculator

Eact Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3337 mL | 11.6686 mL | 23.3372 mL | 46.6744 mL | 58.3431 mL |
| 5 mM | 0.4667 mL | 2.3337 mL | 4.6674 mL | 9.3349 mL | 11.6686 mL |
| 10 mM | 0.2334 mL | 1.1669 mL | 2.3337 mL | 4.6674 mL | 5.8343 mL |
| 50 mM | 0.0467 mL | 0.2334 mL | 0.4667 mL | 0.9335 mL | 1.1669 mL |
| 100 mM | 0.0233 mL | 0.1167 mL | 0.2334 mL | 0.4667 mL | 0.5834 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Larixyl acetate
Catalog No.:BCC8195
CAS No.:4608-49-5
- Interiotherin C
Catalog No.:BCN3636
CAS No.:460090-65-7
- Rucaparib (AG-014699,PF-01367338)
Catalog No.:BCC2207
CAS No.:459868-92-9
- 5-[(2R)-2-Aminopropyl]-2,3-dihydro-1-[3-(phenylmethoxy)propyl]-1H-indole-7-carbonitrile
Catalog No.:BCN1438
CAS No.:459868-73-6
- PEAQX
Catalog No.:BCC5495
CAS No.:459836-30-7
- Obeticholic Acid
Catalog No.:BCC5572
CAS No.:459789-99-2
- SB 452533
Catalog No.:BCC7620
CAS No.:459429-39-1
- JNJ-7777120
Catalog No.:BCC4543
CAS No.:459168-41-3
- SW033291
Catalog No.:BCC3981
CAS No.:459147-39-8
- Boc-Asn-ONp
Catalog No.:BCC3072
CAS No.:4587-33-1
- Ilicic acid
Catalog No.:BCN5505
CAS No.:4586-68-9
- Curcumin
Catalog No.:BCN5504
CAS No.:458-37-7
- Ko 143
Catalog No.:BCC1684
CAS No.:461054-93-3
- Dapagliflozin
Catalog No.:BCC2552
CAS No.:461432-26-8
- 4beta,12-dihydroxyguaian-6,10-diene
Catalog No.:BCN7829
CAS No.:461644-90-6
- Lactulose
Catalog No.:BCC4669
CAS No.:4618-18-2
- Gnemonol B
Catalog No.:BCN3399
CAS No.:462636-74-4
- alpha-Linolenic acid
Catalog No.:BCN8319
CAS No.:463-40-1
- Bay 55-9837
Catalog No.:BCC5932
CAS No.:463930-25-8
- (+)-Borneol
Catalog No.:BCC8376
CAS No.:464-43-7
- (-)-Borneol
Catalog No.:BCC8897
CAS No.:464-45-9
- (-)-Camphor
Catalog No.:BCN7160
CAS No.:464-48-2
- (+)-Camphor
Catalog No.:BCN7161
CAS No.:464-49-3
- Benzopinacol
Catalog No.:BCC8860
CAS No.:464-72-2
Eact, a small molecule activator of TMEM16A, activates TRPV1 and elicits pain- and itch-related behaviours.[Pubmed:26756551]
Br J Pharmacol. 2016 Apr;173(7):1208-18.
BACKGROUND AND PURPOSE: TMEM16A, also known as anoctamin 1 channel, is a member of the Ca(2+)-activated chloride channels family and serves as a heat sensor in the primary nociceptors. Eact is a recently discovered small molecule activator of the TMEM16A channel. Here, we asked if Eact produces pain- and itch-related responses in vivo and investigated the cellular and molecular basis of Eact-elicited responses in dorsal root ganglia (DRG) neurons. EXPERIMENTAL APPROACH: We employed behavioural testing combined with pharmacological inhibition and genetic ablation approaches to identify transient receptor potential vanilloid 1 (TRPV1) as the prominent mediator for Eact-evoked itch- or pain-related responses. We investigated the effects of Eact on TRPV1 and TMEM16A channels expressed in HEK293T cells and in DRG neurons isolated from wild type and Trpv1(-/-) mice using Ca(2+) imaging and patch-clamp recordings. We also used site-directed mutagenesis to determine the molecular basis of Eact activation of TRPV1. KEY RESULTS: Administration of Eact elicited both itch- and pain-related behaviours. Unexpectedly, the Eact-elicited behavioural responses were dependent on the function of TRPV1, as shown by pharmacological inhibition and genetic ablation studies. Eact activated membrane currents and increased intracellular free Ca(2+) in both TRPV1-expressing HEK293T cells and isolated DRG neurons in a TRPV1-dependent manner. Eact activation of the TRPV1 channel was severely attenuated by mutations disrupting the capsaicin-binding sites. CONCLUSIONS AND IMPLICATIONS: Our results suggest that Eact activates primary sensory nociceptors and produces both pain and itch responses mainly through direct activation of TRPV1 channels.
Small-molecule activators of TMEM16A, a calcium-activated chloride channel, stimulate epithelial chloride secretion and intestinal contraction.[Pubmed:21836025]
FASEB J. 2011 Nov;25(11):4048-62.
TMEM16A (ANO1) is a calcium-activated chloride channel (CaCC) expressed in secretory epithelia, smooth muscle, and other tissues. Cell-based functional screening of approximately 110,000 compounds revealed compounds that activated TMEM16A CaCC conductance without increasing cytoplasmic Ca(2+). By patch-clamp, N-aroylaminothiazole "activators" (E(act)) strongly increased Cl(-) current at 0 Ca(2+), whereas tetrazolylbenzamide "potentiators" (F(act)) were not active at 0 Ca(2+) but reduced the EC(50) for Ca(2+)-dependent TMEM16A activation. Of 682 analogs tested, the most potent activator (E(act)) and potentiator (F(act)) produced large and more sustained CaCC Cl(-) currents than general agonists of Ca(2+) signaling, with EC(50) 3-6 muM and Cl(-) conductance comparable to that induced transiently by Ca(2+)-elevating purinergic agonists. Analogs of activators were identified that fully inhibited TMEM16A Cl(-) conductance, providing further evidence for direct TMEM16A binding. The TMEM16A activators increased CaCC conductance in human salivary and airway submucosal gland epithelial cells, and IL-4 treated bronchial cells, and stimulated submucosal gland secretion in human bronchi and smooth muscle contraction in mouse intestine. Small-molecule, TMEM16A-targeted activators may be useful for drug therapy of cystic fibrosis, dry mouth, and gastrointestinal hypomotility disorders, and for pharmacological dissection of TMEM16A function.


