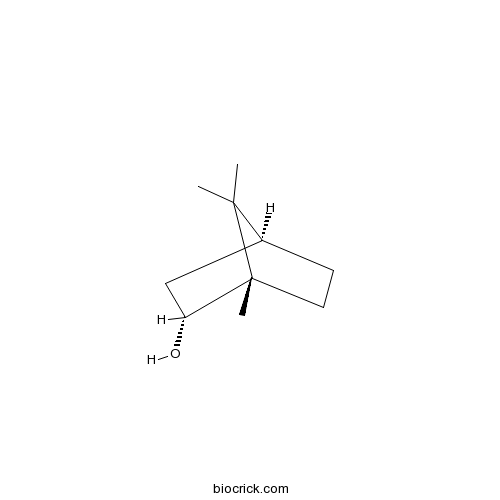
(-)-BorneolCAS# 464-45-9 |
- Isoborneol
Catalog No.:BCN7158
CAS No.:124-76-5
- (+)-Borneol
Catalog No.:BCC8376
CAS No.:464-43-7
- Borneol
Catalog No.:BCN4964
CAS No.:507-70-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 464-45-9 | SDF | Download SDF |
| PubChem ID | 1201518 | Appearance | White powder |
| Formula | C10H18O | M.Wt | 154 |
| Type of Compound | Isoprenoids | Storage | Desiccate at -20°C |
| Synonyms | L-Borneol; (-)-endo-Borneol; Camphyl alcohol; Linderol; Ngai camphor | ||
| Solubility | Soluble in chloroform | ||
| Chemical Name | (1S,3R,4S)-4,7,7-trimethylbicyclo[2.2.1]heptan-3-ol | ||
| SMILES | CC1(C2CCC1(C(C2)O)C)C | ||
| Standard InChIKey | DTGKSKDOIYIVQL-QXFUBDJGSA-N | ||
| Standard InChI | InChI=1S/C10H18O/c1-9(2)7-4-5-10(9,3)8(11)6-7/h7-8,11H,4-6H2,1-3H3/t7-,8+,10+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

(-)-Borneol Dilution Calculator

(-)-Borneol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.4935 mL | 32.4675 mL | 64.9351 mL | 129.8701 mL | 162.3377 mL |
| 5 mM | 1.2987 mL | 6.4935 mL | 12.987 mL | 25.974 mL | 32.4675 mL |
| 10 mM | 0.6494 mL | 3.2468 mL | 6.4935 mL | 12.987 mL | 16.2338 mL |
| 50 mM | 0.1299 mL | 0.6494 mL | 1.2987 mL | 2.5974 mL | 3.2468 mL |
| 100 mM | 0.0649 mL | 0.3247 mL | 0.6494 mL | 1.2987 mL | 1.6234 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-Borneol
Catalog No.:BCC8376
CAS No.:464-43-7
- Bay 55-9837
Catalog No.:BCC5932
CAS No.:463930-25-8
- alpha-Linolenic acid
Catalog No.:BCN8319
CAS No.:463-40-1
- Gnemonol B
Catalog No.:BCN3399
CAS No.:462636-74-4
- Lactulose
Catalog No.:BCC4669
CAS No.:4618-18-2
- 4beta,12-dihydroxyguaian-6,10-diene
Catalog No.:BCN7829
CAS No.:461644-90-6
- Dapagliflozin
Catalog No.:BCC2552
CAS No.:461432-26-8
- Ko 143
Catalog No.:BCC1684
CAS No.:461054-93-3
- Eact
Catalog No.:BCC6313
CAS No.:461000-66-8
- Larixyl acetate
Catalog No.:BCC8195
CAS No.:4608-49-5
- Interiotherin C
Catalog No.:BCN3636
CAS No.:460090-65-7
- Rucaparib (AG-014699,PF-01367338)
Catalog No.:BCC2207
CAS No.:459868-92-9
- (-)-Camphor
Catalog No.:BCN7160
CAS No.:464-48-2
- (+)-Camphor
Catalog No.:BCN7161
CAS No.:464-49-3
- Benzopinacol
Catalog No.:BCC8860
CAS No.:464-72-2
- Arenobufagin
Catalog No.:BCN5401
CAS No.:464-74-4
- Quinamine
Catalog No.:BCN6590
CAS No.:464-85-7
- Conquinamine
Catalog No.:BCN6622
CAS No.:464-86-8
- Asiatic acid
Catalog No.:BCN5506
CAS No.:464-92-6
- Pseudotaraxasterol
Catalog No.:BCN5507
CAS No.:464-98-2
- Arjunolic acid
Catalog No.:BCN5508
CAS No.:465-00-9
- Germanicol
Catalog No.:BCN7507
CAS No.:465-02-1
- Gamabufotalin
Catalog No.:BCN2358
CAS No.:465-11-2
- Neritaloside
Catalog No.:BCN5509
CAS No.:465-13-4
Improved Oral Absorption of Poorly Soluble Curcumin via the Concomitant Use of Borneol.[Pubmed:30903519]
AAPS PharmSciTech. 2019 Mar 22;20(4):150.
In this study, borneol, a natural active compound was applied to improve the bioavailability of curcumin (CUR). In order to increase CUR solubility and dissolution, solid dispersions (SDs) were prepared with the matrix of polyvinylpyrrolidone (PVP) at various ratios by solvent evaporation method. CUR was evidenced to exist as amorphous state in solid dispersion by differential scanning calorimetry (DSC) and powder X-ray diffraction (PXRD). Fourier-transform infrared spectroscopy (FT-IR) was utilized to confirm intermolecular hydrogen bonding. The SD at the ratio of 1:3 (CUR:PVP) exhibited the optimal solubility and dissolution rate in various media. The results of ex vivo permeability studies by everted gut sac method showed that the apparent permeability coefficients (Papp) of CUR in SD across the duodenum, jejunum, and ileum had been significantly improved by co-incubation of borneol, and the improvement degree relied on the concentration of borneol. The pharmacokinetic results in rats indicated that the AUC0-t of CUR-SD (40 mg/kg) co-administration of borneol (90 mg/kg) were 2.53-fold higher than CUR-SD alone, and 19.41-fold higher than pure CUR (200 mg/kg) with borneol (90 mg/kg). Therefore, the combination of borneol and solid dispersion strategy provide a potential approach to enhance the oral bioavailability of CUR.
Borneol for Regulating the Permeability of the Blood-Brain Barrier in Experimental Ischemic Stroke: Preclinical Evidence and Possible Mechanism.[Pubmed:30863478]
Oxid Med Cell Longev. 2019 Feb 4;2019:2936737.
Borneol, a natural product in the Asteraceae family, is widely used as an upper ushering drug for various brain diseases in many Chinese herbal formulae. The blood-brain barrier (BBB) plays an essential role in maintaining a stable homeostatic environment, while BBB destruction and the increasing BBB permeability are common pathological processes in many serious central nervous system (CNS) diseases, which is especially an essential pathological basis of cerebral ischemic injury. Here, we aimed to conduct a systematic review to assess preclinical evidence of borneol for experimental ischemic stroke as well as investigate in the possible neuroprotective mechanisms, which mainly focused on regulating the permeability of BBB. Seven databases were searched from their inception to July 2018. The studies of borneol for ischemic stroke in animal models were included. RevMan 5.3 was applied for data analysis. Fifteen studies investigated the effects of borneol in experimental ischemic stroke involving 308 animals were ultimately identified. The present study showed that the administration of borneol exerted a significant decrease of BBB permeability during cerebral ischemic injury according to brain Evans blue content and brain water content compared with controls (P < 0.01). In addition, borneol could improve neurological function scores (NFS) and cerebral infarction area. Thus, borneol may be a promising neuroprotective agent for cerebral ischemic injury, largely through alleviating the BBB disruption, reducing oxidative reactions, inhibiting the occurrence of inflammation, inhibiting apoptosis, and improving the activity of lactate dehydrogenase (LDH) as well as P-glycoprotein (P-GP) and NO signaling pathway.
Design, characterization and comparison of transdermal delivery of colchicine via borneol-chemically-modified and borneol-physically-modified ethosome.[Pubmed:30744424]
Drug Deliv. 2019 Dec;26(1):70-77.
Gout is a kind of joint disease characterized by the accumulation of monosodium urate (MSU) crystals in the joint and its surrounding tissue, causing persistent hyperuricemia. Colchicine is the first choice of treatment for acute gout attacks. Due to strong toxicity of colchicines oral tablets, there are high fluctuations of blood drug concentration and serious irritation of gastrointestinal tract, and hence a transdermal preparation can help to slow down the blood drug concentration, reduce the frequency of drug taking, and improve the patients' compliance of the drug. The ethosome is a lipid carrier with high concentration of ethanol and has been proved to promote the penetration of drugs into the skin. Borneol (BO) is an excellent penetration enhancer in Chinese medicine, which can promote the entry of drugs into the skin. This paper prepared the borneol-physically-modified colchicine ethosome (COL-bpES) and used the prepared borneol-dioleoyl phosphoethanloamine (BO-DOPE) to prepare borneol-chemically-modified colchicine ethosome (COL-bcES). Compared to the free colchicine aqueous solution (free COL) and normal colchicine ethosome (COL-ES), the borneol-modified colchicine ethosome (COL-bES) demonstrated better drug penetration effect, while the particle size of the COL-bcES was lower than that of the COL-bpES. Toxicity, in vitro diffusion, pharmacokinetics and pharmacodynamics are superior to those of COL-bpES, providing a better delivery system for the treatment of small molecule inflammatory drugs.
Development and validation an LC-MS/MS method to quantify (+)-borneol in rat plasma: Application to a pharmacokinetic study.[Pubmed:30743141]
J Chromatogr B Analyt Technol Biomed Life Sci. 2019 Mar 1;1109:121-127.
(+)-Borneol, a bicyclic monoterpene, has been shown to possess valuable biological properties and potential as a pharmaceutical agent due to anti-inflammatory, anti-oxidant and GABA receptor-enhancing functions; it also enhances the permeability of the blood brain barrier to improve the efficacy of CNS drugs. In this study, we have developed a simple, selective, and rapid liquid chromatography-tandem mass spectrometry method for the assay of (+)-borneol in rat plasma. Verapamil was used as an internal standard. Plasma samples were deproteinized using methanol. The analyte was detected by a mass spectrometer with positive atmospheric pressure chemical ionization by multiple reaction monitoring mode for transitions at m/z [M+H](+) 137.2-->81.0 for (+)-borneol and 455.2-->165.1 for verapamil. The method has been fully validated to ensure good selectivity, a satisfactory lower limit of quantification at 10.0ng/mL, acceptable intra- and inter-day accuracy, and high precision. The method was used for the pharmacokinetic evaluation of (+)-borneol in Sprague-Dawley rats after intravenous, oral, and sublingual administration. The results indicate that oral bioavailability of (+)-borneol was extremely low but sublingual administration yielded rapid absorption and favorable bioavailability of (+)-borneol.
The Effect of Lavandula Stoechas on Toxigenesis and the Growth of Vibrio Parahaemolyticus.[Pubmed:30697296]
Iran J Pathol. 2018 Spring;13(2):245-255. Epub 2018 Jul 17.
Background & objective: Outbreak of food-borne diseases has become more and more important these days and using natural food preservers with high durability is under debate. Vegetative essence is a type of food preserver and many studies have been performed on their antimicrobial effects. The purpose of this study wasto investigate antibacterial effects of lavender essence on toxigenesis and the growth of Vibrio Parahaemolyticus. Methods: lavender essence was prepared and its components were identified using GCMS. Determining minimum interceptorgrowth of Vibrio parahaemolyticus was assessed in test tubes containing BHI. Thermal resistant hemolysin was measured by Kap-RPLAkit. Growth diagram was prepared after determining toxin formation titration of the bacterium during 0, 2, 3, 4, 6, 8 and 24 hours. Results: Cineol, Borneol, Camphor, LinaloolL and Alpha-pinen had the highest concentrations in the essence, respectively. Results of minimum intercepter concentration of lavender (0, 0.005, 0.015, 0.03 and 0.045 percent) on Vibrio parahaemolyticus showed that 0.03% and higher concentrations had the ability to prevent growth and toxin formation of Vibrio parahaemolyticus. In addition, the effect of different concentrations of essence on toxin titration of bacterium showed no toxin at concentrations of 0.030 and 0.045. Conclusion: lavender essence was able to prevent the growth and toxin formation of Vibrio parahaemolyticus.
Phytochemical composition and biological activities of native and in vitro-propagated Micromeria croatica (Pers.) Schott (Lamiaceae).[Pubmed:30666408]
Planta. 2019 Jan 21. pii: 10.1007/s00425-018-03071-5.
MAIN CONCLUSION: In vitro culture conditions and kinetin induced quantitative modifications in the production of the major volatile constituents in Micromeria croatica plantlets. Antimicrobial activity of methanolic extracts obtained from micropropagated and wild-growing plants was evaluated. Micromeria spp. are aromatic plants, many of which were shown to exhibit various biological effects. The present study aimed to determine the content and the composition of the essential oil of in vitro-cultured Micromeria croatica (Pers.) Schott and to evaluate the in vitro antimicrobial activity of its methanolic extract, in order to compare its phytochemical profile and biological activity with wild-growing plants. Shoots regenerated on MS medium without plant growth regulators (PGRs) or supplemented with kinetin were used for phytochemical analysis. Essential oils from both native plant material and in vitro-cultivated M. croatica plants, with a total of 44 identified constituents, were dominated by oxygenated monoterpenes. Borneol was the main component detected in wild-growing plants (25.28%) and micropropagated plants grown on PGR-free medium (20.30%). Kinetin treatment led to increased oil yield and favored the production of oxygenated monoterpenes, dominated by geranial (33.53%) and cis-p-mentha-1(7),8-dien-ol (23.69%). The percentage of total sesquiterpenoids in micropropagated plant material was considerably lower than in wild-growing plants. In vitro culture conditions and PGRs affected the production of essential oils, inducing quantitative modifications in the production of the major volatile constituents in M. croatica plantlets. The antimicrobial activity of M. croatica methanolic extracts was investigated using the broth microdilution method. Extracts obtained from in vitro cultures generally exhibited greater antibacterial potential, compared to wild-growing plants. Among six bacterial strains tested, Bacillus cereus and Staphylococcus aureus were the most sensitive microorganisms. The present study provided evidence that in vitro culture conditions might favorably affect the antimicrobial activity of M. croatica methanolic extracts.


