NeritalosideCAS# 465-13-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 465-13-4 | SDF | Download SDF |
| PubChem ID | 10055 | Appearance | Powder |
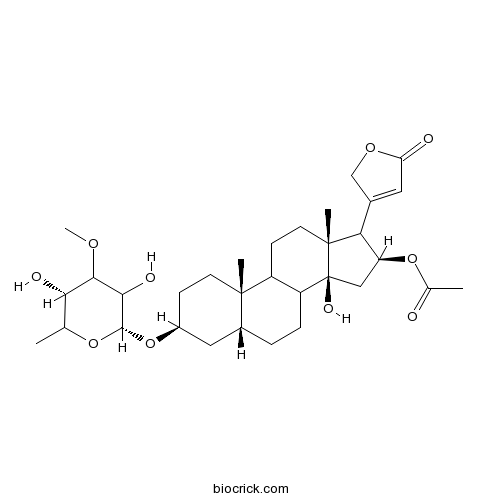
| Formula | C32H48O10 | M.Wt | 592.7 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(3S,5R,10S,13R,14S,16S)-3-[(2R,5S)-3,5-dihydroxy-4-methoxy-6-methyloxan-2-yl]oxy-14-hydroxy-10,13-dimethyl-17-(5-oxo-2H-furan-3-yl)-1,2,3,4,5,6,7,8,9,11,12,15,16,17-tetradecahydrocyclopenta[a]phenanthren-16-yl] acetate | ||
| SMILES | CC1C(C(C(C(O1)OC2CCC3(C(C2)CCC4C3CCC5(C4(CC(C5C6=CC(=O)OC6)OC(=O)C)O)C)C)O)OC)O | ||
| Standard InChIKey | UZQOZJNEDXAJEZ-PBCLCOBXSA-N | ||
| Standard InChI | InChI=1S/C32H48O10/c1-16-26(35)28(38-5)27(36)29(40-16)42-20-8-10-30(3)19(13-20)6-7-22-21(30)9-11-31(4)25(18-12-24(34)39-15-18)23(41-17(2)33)14-32(22,31)37/h12,16,19-23,25-29,35-37H,6-11,13-15H2,1-5H3/t16?,19-,20+,21?,22?,23+,25?,26+,27?,28?,29+,30+,31-,32+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Neritaloside is a cardiac glycoside. 2. Neritaloside exhibits central nervous system depressant activity in mice at a dose of 25 mg/kg. |
| Targets | Sodium Channel | ATPase | Potassium Channel |

Neritaloside Dilution Calculator

Neritaloside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6872 mL | 8.436 mL | 16.8719 mL | 33.7439 mL | 42.1799 mL |
| 5 mM | 0.3374 mL | 1.6872 mL | 3.3744 mL | 6.7488 mL | 8.436 mL |
| 10 mM | 0.1687 mL | 0.8436 mL | 1.6872 mL | 3.3744 mL | 4.218 mL |
| 50 mM | 0.0337 mL | 0.1687 mL | 0.3374 mL | 0.6749 mL | 0.8436 mL |
| 100 mM | 0.0169 mL | 0.0844 mL | 0.1687 mL | 0.3374 mL | 0.4218 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Gamabufotalin
Catalog No.:BCN2358
CAS No.:465-11-2
- Germanicol
Catalog No.:BCN7507
CAS No.:465-02-1
- Arjunolic acid
Catalog No.:BCN5508
CAS No.:465-00-9
- Pseudotaraxasterol
Catalog No.:BCN5507
CAS No.:464-98-2
- Asiatic acid
Catalog No.:BCN5506
CAS No.:464-92-6
- Conquinamine
Catalog No.:BCN6622
CAS No.:464-86-8
- Quinamine
Catalog No.:BCN6590
CAS No.:464-85-7
- Arenobufagin
Catalog No.:BCN5401
CAS No.:464-74-4
- Benzopinacol
Catalog No.:BCC8860
CAS No.:464-72-2
- (+)-Camphor
Catalog No.:BCN7161
CAS No.:464-49-3
- (-)-Camphor
Catalog No.:BCN7160
CAS No.:464-48-2
- (-)-Borneol
Catalog No.:BCC8897
CAS No.:464-45-9
- Oleandrin
Catalog No.:BCN5511
CAS No.:465-16-7
- Polyporenic acid C
Catalog No.:BCN3645
CAS No.:465-18-9
- Bufalin
Catalog No.:BCN1046
CAS No.:465-21-4
- Resibufogenin
Catalog No.:BCN5366
CAS No.:465-39-4
- Quinovic acid
Catalog No.:BCN5512
CAS No.:465-74-7
- Marrubiin
Catalog No.:BCC8208
CAS No.:465-92-9
- Hederagenin
Catalog No.:BCN5513
CAS No.:465-99-6
- alpha-Spinasterol acetate
Catalog No.:BCN5510
CAS No.:4651-46-1
- Cycloartanol
Catalog No.:BCN4860
CAS No.:4657-58-3
- Hederagonic acid
Catalog No.:BCN5514
CAS No.:466-01-3
- Hedragonic acid
Catalog No.:BCN6911
CAS No.:466-02-4
- Proscillaridin A
Catalog No.:BCC8239
CAS No.:466-06-8
Characterization of the anticancer properties of monoglycosidic cardenolides isolated from Nerium oleander and Streptocaulon tomentosum.[Pubmed:21291990]
J Ethnopharmacol. 2011 Apr 12;134(3):781-8.
AIM OF THE STUDY: For identification of the active constituents we investigated the anticancer activity of cardenolides from Streptocaulon tomentosum Wight & Arn. (Asclepiadaceae) and from Nerium oleander L. (Apocynaceae) which are both used against cancer in the traditional medicine in their region of origin. MATERIAL, METHODS AND RESULTS: The antiproliferative activity of cardenolides isolated from roots of Streptocaulon tomentosum (IC(50)<1-15.3 muM after 2 days in MCF7) and of cardenolide containing fractions from the cold aqueous extract of Nerium oleander leaves ("Breastin", mean IC(50) 0.85 mug/ml in a panel of 36 human tumor cell lines), their influence on the cellular viability and on the cell cycle (block at the G2/M-phase or at the S-phase in tumor cells, respectively) were determined using different cell lines. The murine cell line L929 and normal non-tumor cells were not affected. Bioactivity guided fractionation of Breastin resulted in the isolation of the monoglycosidic cardenolides oleandrine, oleandrigeninsarmentoside, Neritaloside, odoroside H, and odoroside A (IC(50)-values between 0.010 and 0.071 mug/ml). CONCLUSIONS: The observed anticancer activities of extracts and isolated cardenolides are in agreement with the ethnomedicinal use of Streptocaulon tomentosum and Nerium oleander. The most active anticancer compounds from both species are monoglycosidic cardenolides possessing the 3beta,14beta-dihydroxy-5beta-card-20(22)-enolide structure with or without an acetoxy group at C-16. The results indicate that the cytotoxic effects are induced by the inhibition of the plasma membrane bound Na(+)/K(+)-ATPase.
LC/MS/MS analyses of an oleander extract for cancer treatment.[Pubmed:10952541]
Anal Chem. 2000 Aug 1;72(15):3547-52.
An HPLC/MS/MS method has been developed for the characterization and quantification of the cardiac glycosides oleandrin, odoroside, Neritaloside and the aglycone oleandrigenin, all contained in a patented-hot-water extract of Nerium oleander L (Anvirzel). Qualitative analysis of such extracts was achieved using a hybrid tandem quadrupole time-of-flight (QqTOF) mass spectrometer. Collision-induced dissociation (CID) mass spectra of oleandrin, oleandrigenin, odoroside, and Neritaloside were obtained with greater than 5 ppm mass accuracy and resolution routinely in excess of 8000 (fwhm). The detection limit for oleandrin of 20 pg (injected) was realized when the precursor-to-product ion transition, m/z 577 --> 373, was monitored. We have also applied the analytical method to the determination of oleandrin, oleandrigenin, Neritaloside, and odoroside in human plasma following an intramuscular injection of Anvirzel.
Bio-active cardenolides from the leaves of Nerium oleander.[Pubmed:9933955]
Phytochemistry. 1999 Feb;50(3):435-8.
A bioactivity directed isolation of the methanolic extract of the fresh, uncrushed leaves of Nerium oleander showing a central nervous system (CNS) depressant effect in mice has been undertaken. As a result, four CNS depressant cardenolides including a new cardenolide, neridiginoside and three known constituents, nerizoside, Neritaloside and odoroside-H, have been isolated which exhibited CNS depressant activity in mice at a dose of 25 mg/kg. The structure of neridiginoside was elucidated as 3 beta-O-(D-diginosyl)-5 beta, 14 beta-dihydroxy-card-20(22)-enolide, using spectroscopic methods including one-dimensional and two-dimensional NMR (COSY-45, NOESY, J-resolved, HMQC and HMBC). The known compounds have been indentified through spectral studies and comparison of data with those reported in the literature.


