PyrosideCAS# 10338-88-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 10338-88-2 | SDF | Download SDF |
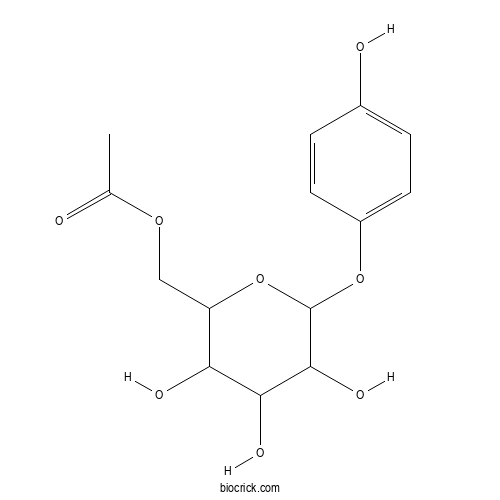
| PubChem ID | 14542705.0 | Appearance | Powder |
| Formula | C14H18O8 | M.Wt | 314.29 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [3,4,5-trihydroxy-6-(4-hydroxyphenoxy)oxan-2-yl]methyl acetate | ||
| SMILES | CC(=O)OCC1C(C(C(C(O1)OC2=CC=C(C=C2)O)O)O)O | ||
| Standard InChIKey | XABUTYXAHYMCDK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H18O8/c1-7(15)20-6-10-11(17)12(18)13(19)14(22-10)21-9-4-2-8(16)3-5-9/h2-5,10-14,16-19H,6H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Pyroside Dilution Calculator

Pyroside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1818 mL | 15.9089 mL | 31.8177 mL | 63.6355 mL | 79.5444 mL |
| 5 mM | 0.6364 mL | 3.1818 mL | 6.3635 mL | 12.7271 mL | 15.9089 mL |
| 10 mM | 0.3182 mL | 1.5909 mL | 3.1818 mL | 6.3635 mL | 7.9544 mL |
| 50 mM | 0.0636 mL | 0.3182 mL | 0.6364 mL | 1.2727 mL | 1.5909 mL |
| 100 mM | 0.0318 mL | 0.1591 mL | 0.3182 mL | 0.6364 mL | 0.7954 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Aljesaconitine B
Catalog No.:BCX1154
CAS No.:101247-24-9
- Spicatine A
Catalog No.:BCX1153
CAS No.:124256-81-1
- Butyl chlorogenate
Catalog No.:BCX1152
CAS No.:132741-56-1
- Butyl neochlorogenate
Catalog No.:BCX1151
CAS No.:409361-64-4
- Methyl brevifolincarboxylate
Catalog No.:BCX1150
CAS No.:154702-76-8
- Rubropunctatin
Catalog No.:BCX1149
CAS No.:514-67-0
- Toralactone
Catalog No.:BCX1148
CAS No.:41743-74-2
- Eicosapentaenoic acid
Catalog No.:BCX1147
CAS No.:10417-94-4
- 24(28)-Dehydroergosterol
Catalog No.:BCX1146
CAS No.:29560-24-5
- Jaligonic acid
Catalog No.:BCX1145
CAS No.:51776-39-7
- Reptoside
Catalog No.:BCX1144
CAS No.:53839-03-5
- Euphornin
Catalog No.:BCX1143
CAS No.:80454-47-3
- Proprotogracillin
Catalog No.:BCX1156
CAS No.:78229-03-5
- Kadsurenone
Catalog No.:BCX1157
CAS No.:95851-37-9
- Neolinustatin
Catalog No.:BCX1158
CAS No.:72229-42-6
- Neotheaflavin
Catalog No.:BCX1159
CAS No.:36451-14-6
- Zingiberene
Catalog No.:BCX1160
CAS No.:495-60-3
- Soyasaponin Af
Catalog No.:BCX1161
CAS No.:117230-32-7
- Soyasaponin Ae
Catalog No.:BCX1162
CAS No.:117230-34-9
- Coronatine
Catalog No.:BCX1163
CAS No.:62251-96-1
- Coronafacic acid
Catalog No.:BCX1164
CAS No.:62251-98-3
- Aucubigenin
Catalog No.:BCX1165
CAS No.:64274-28-8
- 6-methoxy-bispyranoxanthone
Catalog No.:BCX1166
CAS No.:115713-10-5
- Hispolon
Catalog No.:BCX1167
CAS No.:173933-40-9
Qualitative and quantitative chromatographic investigation of hydroquinone derivatives in Pyrus communis L. flowers.[Pubmed:14714860]
Acta Pol Pharm. 2003 Jul-Aug;60(4):309-12.
The qualitative analysis and quantitative determination of hydroquinone derivatives (arbutin, Pyroside) in the flowers of naturally growing pear tree and of its four cultivated varieties (Pomaranczowka, Lukasowka, Klapsa, Salisbury) were carried out. The comparative qualitative analysis of hydroquinone derivatives was investigated chromatographically (TLC). Arbutin and Pyroside were found in all the studied plant materials and free hydroquinone was found in buds of naturally growing pear. The content of arbutin and Pyroside was determined by the HPLC method.
The glycosidic precursor of (Z)-5-ethylidene-2(5H)-furanone in Halocarpus biformis juvenile foliage.[Pubmed:8688176]
Phytochemistry. 1996 May;42(2):453-9.
A new glycosidic lactone, (5R,6R)-5-(1-hydroxyethyl)-2(5H)-furanone beta-D-glucopyranoside, has been identified as the principal precursor of (Z)-5-ethylidene-2(5H)-furanone in juvenile foliage of the New Zealand tree Halocarpus biformis. Three related lactone glycosides were isolated in smaller amounts, together with the known phenolic glycosides Pyroside, arbutin and picein. The principal lactone glycoside underwent facile elimination of glucose, in neutral or basic conditions, to yield (Z)-5-ethylidene-2(5H)-furanone and its E-isomer. This lactone glycoside was also detected in foliage of H. bidwillii and H. kirkii.


