ToralactoneCAS# 41743-74-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

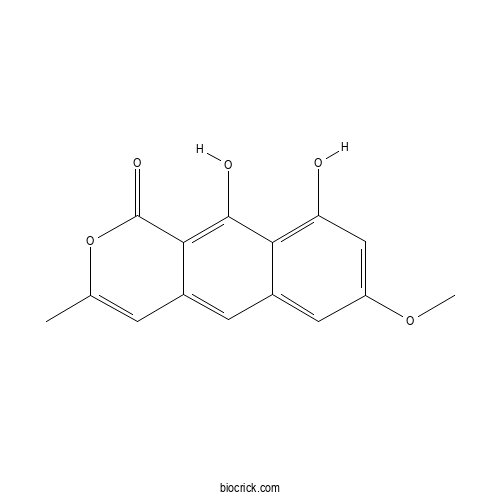
| Cas No. | 41743-74-2 | SDF | Download SDF |
| PubChem ID | 5321980.0 | Appearance | Powder |
| Formula | C15H12O5 | M.Wt | 272.26 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 9,10-dihydroxy-7-methoxy-3-methylbenzo[g]isochromen-1-one | ||
| SMILES | CC1=CC2=CC3=CC(=CC(=C3C(=C2C(=O)O1)O)O)OC | ||
| Standard InChIKey | WEHXAEGTVPWKDY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H12O5/c1-7-3-8-4-9-5-10(19-2)6-11(16)12(9)14(17)13(8)15(18)20-7/h3-6,16-17H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Toralactone Dilution Calculator

Toralactone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.673 mL | 18.3648 mL | 36.7296 mL | 73.4592 mL | 91.824 mL |
| 5 mM | 0.7346 mL | 3.673 mL | 7.3459 mL | 14.6918 mL | 18.3648 mL |
| 10 mM | 0.3673 mL | 1.8365 mL | 3.673 mL | 7.3459 mL | 9.1824 mL |
| 50 mM | 0.0735 mL | 0.3673 mL | 0.7346 mL | 1.4692 mL | 1.8365 mL |
| 100 mM | 0.0367 mL | 0.1836 mL | 0.3673 mL | 0.7346 mL | 0.9182 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Eicosapentaenoic acid
Catalog No.:BCX1147
CAS No.:10417-94-4
- 24(28)-Dehydroergosterol
Catalog No.:BCX1146
CAS No.:29560-24-5
- Jaligonic acid
Catalog No.:BCX1145
CAS No.:51776-39-7
- Reptoside
Catalog No.:BCX1144
CAS No.:53839-03-5
- Euphornin
Catalog No.:BCX1143
CAS No.:80454-47-3
- Hydroxypropyl tetrahydropyrantriol
Catalog No.:BCX1142
CAS No.:439685-79-7
- Presenegenin
Catalog No.:BCX1141
CAS No.:2163-40-8
- Ligustrosidic acid
Catalog No.:BCX1140
CAS No.:96382-89-7
- 3α-Hydroxymogrol
Catalog No.:BCX1139
CAS No.:1343402-73-2
- N-methyltyramine
Catalog No.:BCX1138
CAS No.:370-98-9
- Ganosporeric acid A
Catalog No.:BCX1137
CAS No.:135357-25-4
- Avenanthramide A
Catalog No.:BCX1136
CAS No.:108605-70-5
- Rubropunctatin
Catalog No.:BCX1149
CAS No.:514-67-0
- Methyl brevifolincarboxylate
Catalog No.:BCX1150
CAS No.:154702-76-8
- Butyl neochlorogenate
Catalog No.:BCX1151
CAS No.:409361-64-4
- Butyl chlorogenate
Catalog No.:BCX1152
CAS No.:132741-56-1
- Spicatine A
Catalog No.:BCX1153
CAS No.:124256-81-1
- Aljesaconitine B
Catalog No.:BCX1154
CAS No.:101247-24-9
- Pyroside
Catalog No.:BCX1155
CAS No.:10338-88-2
- Proprotogracillin
Catalog No.:BCX1156
CAS No.:78229-03-5
- Kadsurenone
Catalog No.:BCX1157
CAS No.:95851-37-9
- Neolinustatin
Catalog No.:BCX1158
CAS No.:72229-42-6
- Neotheaflavin
Catalog No.:BCX1159
CAS No.:36451-14-6
- Zingiberene
Catalog No.:BCX1160
CAS No.:495-60-3
Exploring the mechanism of Cassiae semen in regulating lipid metabolism through network pharmacology and experimental validation.[Pubmed:36645186]
Biosci Rep. 2023 Feb 27;43(2):BSR20221375.
BACKGROUND: Multiple studies have assessed the role of Cassiae semen (CS) in regulating lipid metabolism. However, the mechanism of action of CS on non-alcoholic fatty liver disease (NAFLD) has seen rare scrutiny. OBJECTIVE: The objective of this study was to explore the regulatory mechanism of CS on lipid metabolism in NAFLD. METHODS: Components of CS ethanol extract (CSEE) were analyzed and identified using UPLC-Q-Orbirap HRMS. The candidate compounds of CS and its relative targets were extracted from the Traditional Chinese Medicine Systems Pharmacology, Swiss-Target-Prediction, and TargetNet web server. The Therapeutic Target Database, Genecards, Online Mendelian Inheritance in Man, and DisGeNET were searched for NAFLD targets. Binding affinity between potential core components and key targets was established employing molecular docking simulations. After that, free fatty acid (FFA)-induced HepG2 cells were used to further validate part of the network pharmacology results. RESULTS: Six genes, including Caspase 3 (CASP3), phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PIK3CA), epidermal growth factor receptor (EGFR), and amyloid beta (A4) precursor protein (APP) were identified as key targets. The mitogen-activated protein kinase (MAPK) signaling pathway was found to associate closely with CS's effect on NAFLD. Per molecular docking findings, Toralactone and quinizarin formed the most stable combinations with hub genes. About 0.1 (vs. FFA, P<0.01) and 0.2 (vs. FFA, P<0.05) mg/ml CSEE decreased lipid accumulation in vitro by reversing the up-regulation of CASP3, EGFR, and APP and the down-regulation of PIK3CA. CONCLUSION: CSEE can significantly reduce intracellular lipid accumulation by modulating the MAPK signaling pathway to decrease CASP3 and EGFR expression.
Phytochemistry, Ethnopharmacological Uses, Biological Activities, and Therapeutic Applications of Cassia obtusifolia L.: A Comprehensive Review.[Pubmed:34684833]
Molecules. 2021 Oct 15;26(20):6252.
Cassia obtusifolia L., of the Leguminosae family, is used as a diuretic, laxative, tonic, purgative, and natural remedy for treating headache, dizziness, constipation, tophobia, and lacrimation and for improving eyesight. It is commonly used in tea in Korea. Various anthraquinone derivatives make up its main chemical constituents: emodin, chrysophanol, physcion, obtusifolin, obtusin, au rantio-obtusin, chryso-obtusin, alaternin, questin, aloe-emodin, gluco-aurantio-obtusin, gluco-obtusifolin, naphthopyrone glycosides, Toralactone-9-beta-gentiobioside, Toralactone gentiobioside, and cassiaside. C. obtusifolia L. possesses a wide range of pharmacological properties (e.g., antidiabetic, antimicrobial, anti-inflammatory, hepatoprotective, and neuroprotective properties) and may be used to treat Alzheimer's disease, Parkinson's disease, and cancer. In addition, C. obtusifolia L. contributes to histamine release and antiplatelet aggregation. This review summarizes the botanical, phytochemical, and pharmacological features of C. obtusifolia and its therapeutic uses.
Mechanism of Radix Rhei Et Rhizome Intervention in Cerebral Infarction: A Research Based on Chemoinformatics and Systematic Pharmacology.[Pubmed:34531920]
Evid Based Complement Alternat Med. 2021 Sep 6;2021:6789835.
OBJECTIVE: To explore the therapeutic targets, network modules, and coexpressed genes of Radix Rhei Et Rhizome intervention in cerebral infarction (CI), and to predict significant biological processes and pathways through network pharmacology. To explore the differential proteins of Radix Rhei Et Rhizome intervention in CI, conduct bioinformatics verification, and initially explain the possible therapeutic mechanism of Radix Rhei Et Rhizome intervention in CI through proteomics. METHODS: The TCM database was used to predict the potential compounds of Radix Rhei Et Rhizome, and the PharmMapper was used to predict its potential targets. GeneCards and OMIM were used to search for CI-related genes. Cytoscape was used to construct a protein-protein interaction (PPI) network and to screen out core genes and detection network modules. Then, DAVID and Metascape were used for enrichment analysis. After that, in-depth analysis of the proteomics data was carried out to further explore the mechanism of Radix Rhei Et Rhizome intervention in CI. RESULTS: (1) A total of 14 Radix Rhei Et Rhizome potential components and 425 potential targets were obtained. The core components include sennoside A, palmidin A, emodin, Toralactone, and so on. The potential targets were combined with 297 CI genes to construct a PPI network. The targets shared by Radix Rhei Et Rhizome and CI include ALB, AKT1, MMP9, IGF1, CASP3, etc. The biological processes that Radix Rhei Et Rhizome may treat CI include platelet degranulation, cell migration, fibrinolysis, platelet activation, hypoxia, angiogenesis, endothelial cell apoptosis, coagulation, and neuronal apoptosis. The signaling pathways include Ras, PI3K-Akt, TNF, FoxO, HIF-1, and Rap1 signaling pathways. (2) Proteomics shows that the top 20 proteins in the differential protein PPI network were Syp, Syn1, Mbp, Gap43, Aif1, Camk2a, Syt1, Calm1, Calb1, Nsf, Nefl, Hspa5, Nefh, Ncam1, Dcx, Unc13a, Mapk1, Syt2, Dnm1, and Cltc. Differential protein enrichment results show that these proteins may be related to synaptic vesicle cycle, vesicle-mediated transport in synapse, presynaptic endocytosis, synaptic vesicle endocytosis, axon guidance, calcium signaling pathway, and so on. CONCLUSION: This study combined network pharmacology and proteomics to explore the main material basis of Radix Rhei Et Rhizome for the treatment of CI such as sennoside A, palmidin A, emodin, and Toralactone. The mechanism may be related to the regulation of biological processes (such as synaptic vesicle cycle, vesicle-mediated transport in synapse, presynaptic endocytosis, and synaptic vesicle endocytosis) and signaling pathways (such as Ras, PI3K-Akt, TNF, FoxO, HIF-1, Rap1, and axon guidance).
In Vitro and in Silico Human Monoamine Oxidase Inhibitory Potential of Anthraquinones, Naphthopyrones, and Naphthalenic Lactones from Cassia obtusifolia Linn Seeds.[Pubmed:31592482]
ACS Omega. 2019 Sep 18;4(14):16139-16152.
In recent years, Cassia seed extract has been reported as a neuroprotective agent in various models of neurodegeneration, mainly via an antioxidant mechanism. However, no one has previously reported the effects of Cassia seed extract and its phytochemicals on human monoamine oxidase (hMAO) enzyme activity. The seed methanol extract, the solvent-soluble fractions, and almost all isolated compounds displayed selective inhibition of hMAO-A isozyme activity. Interestingly, compounds obtusin (3), alaternin (8), aloe-emodin (9), questin (12), rubrofusarin (13), cassiaside (15), Toralactone 9-O-beta-gentiobioside (26), and (3S)-9,10-dihydroxy-7-methoxy-3-methyl-1-oxo-3,4-dihydro-1H-benzo[g]isochromene-3-carboxylic acid 9-O-beta-d-glucopyranoside (38) showed the most promising inhibition of the hMAO-A isozyme with IC(50) values of 0.17-11 muM. The kinetic study characterized their mode of inhibition and molecular docking simulation predicted interactions with Ile-335 and Tyr-326 in support of the substrate/inhibitor selectivity in respective isozymes. These results demonstrate that Cassia seed extract and its constituents inhibit hMAO-A enzyme activity with high selectivity and suggest that they could play a preventive role in neurodegenerative diseases, especially anxiety and depression.
Morphological and chemical analyses of Eriocauli Flos sold in Taiwan markets.[Pubmed:28987371]
J Food Drug Anal. 2017 Oct;25(4):939-945.
Eriocauli Flos (Gujingcao; EF), the dried capitulum with the peduncle of Eriocaulon buergerianum Koern. (Eriocaulaceae), is a Chinese herbal medicine for treating eye diseases and inflammation. However, several species of the Eriocaulon genus are used as substitutes in different areas. To examine the species of EF used in Taiwan and to establish the quality control platform, morphological and chemical analyses have been performed. Ten major compounds, including apigenin (7) and its 7-O-beta-D-glucopyranoside (1) and 7-O-(6-O-E-coumaroyl)-beta-D-glucopyranoside (6), hispidulin (8) and its 7-O-beta-D-glucopyranoside (2) and 7-O-(6-O-E-coumaroyl)-beta-D-glucopyranoside (5), jaceosidin (9) and its 7-O-beta-D-glucopyranoside (3), and Toralactone (10) and its 9-O-beta-D-glucopyranosyl(1-->6)-beta-D-glucopyranoside (4), were isolated and identified from commercially available EF. Morphological investigation showed that two kinds of EFs and most of the EFs sold in Taiwan herbal markets are capitulum without the peduncle. A simultaneous high performance liquid chromatography and ultra performance liquid chromatography analyses of multiple components (1-10) in commercially available EFs, collected from different areas of Taiwan, was conducted. Results showed wide variations in morphology and chemical profiles between capitulum with and without the peduncle. In comparison with an authentic E. buergerianum, we found not only the morphology but also the chemical profile was different from both collected samples. In terms of the morphological examination, the samples without peduncle are closer to the authentic one. To ensure the correct EF materia medica is used in Taiwan so as to guarantee their therapeutic efficacy in clinical practice, further monitoring is necessary.
Toralactone glycoside in Cassia obtusifolia mediates hepatoprotection via an Nrf2-dependent anti-oxidative mechanism.[Pubmed:28578058]
Food Res Int. 2017 Jul;97:340-346.
Cassia obtusifolia L. (Leguminosae) seeds are a well-known medicinal food in East Asia and are used to clear liver heat, sharpen vision, lubricate the intestines, and promote bowel movement. The aims of the present study were to identify the hepatoprotective components of C. obtusifolia seeds by bioactivity-guided isolation and to elucidate their mechanisms of action. Ten phenolic glycosides were isolated from the most active ethyl acetate fraction, and their chemical structures were elucidated by spectroscopic analyses. Among the isolated compounds, Toralactone 9-O-gentiobioside (5) had the highest hepatoprotective efficacy against tert-butylhydroperoxide-induced cell death in HepG2 cells. Immunoblotting and real-time polymerase chain reaction analyses revealed that the hepatoprotective effects were exerted through nuclear factor erythroid-2-related factor 2 (Nrf2)-dependent antioxidative signaling. Together, these results provide insights into the effects of this medicinal plant as well as a basis for developing hepatoprotective agents as pharmaceuticals and/or nutraceuticals.
Inhibitory activities of major anthraquinones and other constituents from Cassia obtusifolia against beta-secretase and cholinesterases.[Pubmed:27321278]
J Ethnopharmacol. 2016 Sep 15;191:152-160.
ETHNOPHARMACOLOGICAL RELEVANCE: Semen Cassiae has been traditionally used as an herbal remedy for liver, eye, and acute inflammatory diseases. Recent pharmacological reports have indicated that Cassiae semen has neuroprotective effects, attributable to its anti-inflammatory actions, in ischemic stroke and Alzheimer's disease (AD) models. AIM OF THE STUDY: The basic goal of this study was to evaluate the anti-AD activities of C. obtusifolia and its major constituents. Previously, the extract of C. obtusifolia seeds, was reported to have memory enhancing properties and anti-AD activity to ameliorate amyloid beta-induced synaptic dysfunction. However, the responsible components of C. obtusifolia seeds in an AD are currently still unknown. In this study, we investigated the inhibitory effects of C. obtusifolia and its constituents against acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and beta-site amyloid precursor protein (APP) cleaving enzyme 1 (BACE1) enzyme activity. MATERIALS AND METHODS: In vitro cholinesterase enzyme assays by using AChE, BChE, and BACE1 were performed. We also scrutinized the potentials of Cassiae semen active component as BACE1 inhibitors via enzyme kinetics and molecular docking simulation. RESULTS: In vitro enzyme assays demonstrated that C. obtusifolia and its major constituents have promising inhibitory potential against AChE, BChE, and BACE1. All Cassiae semen constituents exhibited potent inhibitory activities against AChE and BACE1 with IC50 values of 6.29-109microg/mL and 0.94-190microg/mL, whereas alaternin, questin, and Toralactone gentiobioside exhibited significant inhibitory activities against BChE with IC50 values of 113.10-137.74microg/mL. Kinetic study revealed that alaternin noncompetitively inhibited, whereas cassiaside and emodin showed mixed-type inhibition against BACE1. Furthermore, molecular docking simulation results demonstrated that hydroxyl group of alaternin and emodin tightly interacted with the active site residues of BACE1 and their relevant binding energies (-6.62 and -6.89kcal/mol), indicating a higher affinity and tighter binding capacity of these compounds for the active site of BACE1. CONCLUSION: The findings of the present study suggest the potential of C. obtusifolia and its major constituents for use in the development of therapeutic or preventive agents for AD, especially through inhibition of AChE, BChE and BACE1 activities.
Molecular Characterization of the Cercosporin Biosynthetic Pathway in the Fungal Plant Pathogen Cercospora nicotianae.[Pubmed:26938470]
J Am Chem Soc. 2016 Mar 30;138(12):4219-28.
Perylenequinones are a class of photoactivated polyketide mycotoxins produced by fungal plant pathogens that notably produce reactive oxygen species with visible light. The best-studied perylenequinone is cercosporin-a product of the Cercospora species. While the cercosporin biosynthetic gene cluster has been described in the tobacco pathogen Cercospora nicotianae, little is known of the metabolite's biosynthesis. Furthermore, in vitro investigations of the polyketide synthase central to cercosporin biosynthesis identified the naphthopyrone nor-Toralactone as its direct product-an observation in conflict with published biosynthetic proposals. Here, we present an alternative biosynthetic pathway to cercosporin based on metabolites characterized from a series of biosynthetic gene knockouts. We show that nor-Toralactone is the key polyketide intermediate and the substrate for the unusual didomain protein CTB3. We demonstrate the unique oxidative cleavage activity of the CTB3 monooxygenase domain in vitro. These data advance our understanding of perylenequinone biosynthesis and expand the biochemical repertoire of flavin-dependent monooxygenases.
A new comprehensive procedure for the quality control of Semen Cassiae and its application in evaluating commercially available material in China.[Pubmed:23845556]
Chin J Nat Med. 2013 Jul;11(4):433-41.
AIM: To establish a more comprehensive and suitable procedure for the quality control of Semen Cassiae which can be used to supplement the evaluation procedure adopted by the Pharmacopoeia of the Peoples Republic of China. METHODS: A HPLC assay-based comprehensive quality evaluation procedure for Semen Cassiae using three bioactive compounds including anthraquinones and naphthopyrones, i.e., chrysophanol-1-O-beta-D-glucopyranosyl-(1-->3)-beta-D-glucopyranosyl-(1-->6)-beta-D-glucopyranoside (1), rubrofusarin-6-O-beta-D-gentiobioside (2) and Toralactone-9-O-beta-D-gentiobioside (3) as the index components was established. The resultant data were further analyzed by principal component analysis (PCA) and data distribution methods using software SPSS 16.0. RESULTS: Sixty-six batches of Semen Cassiae obtained from various regions of China were analyzed with the procedure. Based on the test results of these batches, the content limits of the three bioactive compounds in Semen Cassiae were proposed. CONCLUSION: The procedure established herein is more comprehensive and appropriate for the quality evaluation of Semen Cassiae commercially available in China, and can be used as a useful supplement to the current official method in China for the quality evaluation of Semen Cassiae.
Structure elucidation of a sodium salified anthraquinone from the seeds of Cassia obtusifolia by NMR technique assisted with acid-alkali titration.[Pubmed:21761451]
Magn Reson Chem. 2011 Aug;49(8):529-32.
A new sodium salt of anthraquinone named sodium emodin-1-O-beta-gentiobioside, together with nine known compounds, viz. rubrofusarin-6-O-beta-D-gentiobioside, chrysophanol-1-O-beta-D-glucopyranosyl-(1-3)-beta-D-glucopyranosyl-(1-6)-beta-D-glucopyranoside, obtusifolin-2-O-beta-D-glucopyranoside, aurantio-obtusin-6-O-beta-D-glucopyranoside, physcion-8-O-beta-D-glucopyranoside, 1-hydroxyl-2-acetyl-3,8-dimethoxy-6-O-beta-D-apiofuranosyl-(1-2)-beta-D-glucosylnaphthalene, Toralactone-9-O-beta-D-gentiobioside, aurantio-obtusin, rubrofusarin-6-O-beta-D-apiofuranosyl-(1-6)-O-beta-D-glucopyranoside, was isolated from the seeds of Cassia obtusifolia and its structure was elucidated by (1)H and (13)C NMR technique assisted with acid-alkali titration. The change of chemical shifts of sodium emodin-1-O-beta-gentiobioside before and after acid-alkali titration was also characterized.
Extract of Cassiae Semen and its major compound inhibit S100b-induced TGF-beta1 and fibronectin expression in mouse glomerular mesangial cells.[Pubmed:20483351]
Eur J Pharmacol. 2010 Sep 1;641(1):7-14.
Non-enzymatic glycation reactions between reducing sugar and free reactive amino groups of protein lead to the formation of advanced glycation end products, which increase under conditions of aging or diabetes. A previous study showed that extracts of Cassiae Semen (CS), the seed of Cassia tora, had inhibitory activity on advanced glycation end products formation in vitro. To examine the pharmacological effects of a butanol-soluble extract of CS under conditions of diabetic nephropathy, we evaluated the expression of transforming growth factor-beta1 (TGF-beta1) and fibronectin, key mediators of diabetic nephropathy, in mouse glomerular mesangial cells cultured in the presence of S100b (a specific ligand for receptor of advanced glycation end products). CS inhibited S100b-induced TGF-beta1 and fibronectin expression in mouse mesangial cells by suppressing activation of Smad2/3, extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK), and oxidative stress. Moreover, CS suppressed nuclear factor-kappa B (NF-kappaB) activation in S100b-stimulated mouse mesangial cells. To identify the active compounds of CS, three major compounds, rubrofusarin-6-O-beta-d-gentiobioside (CS-A), Toralactone-9-O-beta-d-gentiobioside (CS-B), and cassiaside (CS-C), were tested in cells. Of these compounds, CS-A significantly decreased the expression of TGF-beta1 and fibronectin and NF-kappaB DNA binding activity. These findings suggest that CS, especially CS-A, has potential as a preventive agent for advanced glycation end products-related diabetic complications.
Inhibitory activities of Cassia tora and its anthraquinone constituents on angiotensin-converting enzyme.[Pubmed:18803227]
Phytother Res. 2009 Feb;23(2):178-84.
As a component of our program that pertains to the isolation of antihypertensive agents derived from natural products, we screened the bioactivity of seeds from raw and roasted Cassia tora via angiotensin converting enzyme (ACE) inhibitory assays. We found that both of the MeOH extracts from the raw and roasted C. tora exhibited significant inhibitory properties against ACE, demonstrating more than 50% inhibition at a concentration of 163.93 microg/mL. Emodin (3), alaternin (4), gluco-obtusifolin (5), cassiaside (6), gluco-aurantioobtusin (7), cassitoroside (8), Toralactone gentiobioside (9), and chrysophanol triglucoside (10) had been previously isolated; however, questin (1) and 2-hydroxyemodin 1-methylether (2) were isolated from C. tora for the first time in this study. Among them, only anthraquinone glycoside (7) demonstrated marked inhibitory activity against ACE, with an IC(50) value of 30.24 +/- 0.20 microM. Conversely, aurantioobtusin (7a), obtained from the acid hydrolysis of 7, showed no activity. Further inhibitory kinetics analyzed from Lineweaver-Burk plots showed 7 to be a competitive inhibitor with a Ki value of 8.3 x 10(-5) M. Moreover, compound 7 showed marked inhibitory and scavenging activities with an IC(50) value of 49.64 +/- 0.37 microM (positive control; trolox: 26.07 +/- 1.05 microM) for total reactive oxygen species generation, and 4.60 +/- 1.12 microM (positive control; penicillamine: 0.24 +/- 0.04 microM) for ONOO(-).
Antibacterial phenolic components from Eriocaulon buergerianum.[Pubmed:18191163]
Phytochemistry. 2008 Mar;69(5):1279-86.
Five phenolic components, 1,3,6-trihydroxy-2,5,7-trimethoxyxanthone (1), 7,3'-dihydroxy-5,4',5'-trimethoxyisoflavone (2), Toralactone-9-O-beta-D-glucopyranoside (3), patuletin-3-O-[2-O-E-feruloyl-beta-d-glucopyranosyl-(1-->6)-beta-d-glucopyranoside] (4), patuletin-3-O-[beta-d-glucopyranosyl-(1-->3)-2-O-E-caffeoyl-beta-D-glucopyranosyl-(1-->6)-beta-D-glucopyranoside] (5), along with 19 known compounds were isolated from Eriocaulon buergerianum (Eriocaulaceae). Their structures were determined by spectroscopic and chemical methods. All 24 isolated compounds were tested against the pathogenic bacteria Staphylococcus aureus (ATCC 25923); as a result, 10 compounds were found to exhibit antibacterial activity with MICs ranging from 32 to 256 microg/ml.
Estrogenic and anti-estrogenic activities of Cassia tora phenolic constituents.[Pubmed:17917292]
Chem Pharm Bull (Tokyo). 2007 Oct;55(10):1476-82.
Through an estrogenic activity bioassay-guided fractionation of the 70% ethanolic extract of Cassia tora seeds two new phenolic triglucosides, torachrysone 8-O-[beta-D-glucopyranosyl(1-->3)-O-beta-D-glucopyranosyl(1-->6)-O-beta-D-glucopyranoside] (1) and Toralactone 9-O-[beta-D-glucopyranosyl-(1-->3)-O-beta-D-glucopyranosyl-(1-->6)-O-beta-D-glucopyranoside] (2), along with seven known compounds were isolated. The structures of the new compounds were elucidated on the basis of spectroscopic and chemical evidence. The estrogenic activity of the fractions and the isolated compounds were investigated using the estrogen-dependent proliferation of MCF-7 cells. In addition, the yeast two hybrid assay expressing estrogen receptor alpha (ERalpha) and beta (ERbeta) and the ERalpha competitor screening assay (ligand binding screen) were used to verify the binding affinities of the isolated compounds to ER. Furthermore, a naringinase pre-treatment of the 70% alcoholic extract of Cassia tora seeds resulted in a significant increase in its estrogenic activity. From the naringinase pre-treated extract six compounds were isolated, among which 6-hydroxymusizin and aurantio-obtusin showed the most potent estrogenic activity, while torachrysone, rubrofusarin and Toralactone showed a significant anti-estrogenic activity. Finally, the structure requirements responsible for the estrogenic activity of the isolated compounds were studied by investigating the activity of several synthetic compounds and chemically modifying the isolated compounds. The basic nucleus 1,3,8-trihyroxynaphthalene (T(3)HN) was found to play a principal role in the binding affinity of these compounds to ER.
Naphthopyrone glucosides from the seeds of Cassia tora with inhibitory activity on advanced glycation end products (AGEs) formation.[Pubmed:16903080]
Arch Pharm Res. 2006 Jul;29(7):587-90.
Three naphthopyrone glucosides, cassiaside (1), rubrofusarin-6-O-beta-D-gentiobioside (2), and Toralactone-9-O-beta-D-gentiobioside (3), were isolated from the BuOH-soluble extract of the seeds of Cassia tora as active constituents, using an in vitro bioassay based on the inhibition of advanced glycation end products (AGEs) to monitor chromatographic fractionation. The structures of 1-3 were determined by spectroscopic data interpretation, particularly by extensive 1D and 2D NMR studies. All the isolates (1-3) were evaluated for the inhibitory activity on AGEs formation in vitro.


