EuphorninCAS# 80454-47-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 80454-47-3 | SDF | Download SDF |
| PubChem ID | 6440619.0 | Appearance | Powder |
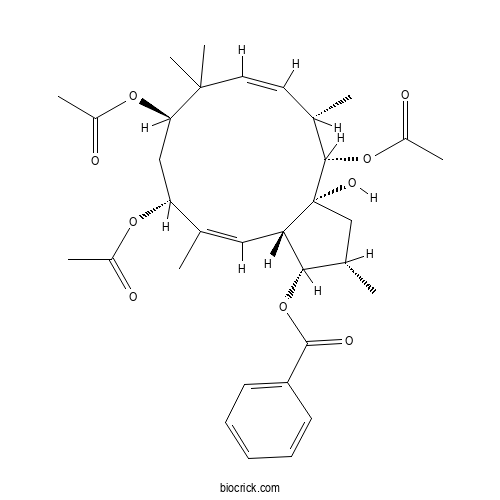
| Formula | C33H44O9 | M.Wt | 584.71 |
| Type of Compound | Terpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1S,2S,3aR,4S,5S,6Z,9R,11R,12Z,13aS)-4,9,11-triacetyloxy-3a-hydroxy-2,5,8,8,12-pentamethyl-2,3,4,5,9,10,11,13a-octahydro-1H-cyclopenta[12]annulen-1-yl] benzoate | ||
| SMILES | CC1CC2(C(C1OC(=O)C3=CC=CC=C3)C=C(C(CC(C(C=CC(C2OC(=O)C)C)(C)C)OC(=O)C)OC(=O)C)C)O | ||
| Standard InChIKey | BRVXVMOWTHQKHC-NHTJSLHDSA-N | ||
| Standard InChI | InChI=1S/C33H44O9/c1-19-14-15-32(7,8)28(40-23(5)35)17-27(39-22(4)34)20(2)16-26-29(42-31(37)25-12-10-9-11-13-25)21(3)18-33(26,38)30(19)41-24(6)36/h9-16,19,21,26-30,38H,17-18H2,1-8H3/b15-14-,20-16-/t19-,21-,26-,27+,28+,29-,30-,33+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Euphornin Dilution Calculator

Euphornin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7102 mL | 8.5512 mL | 17.1025 mL | 34.205 mL | 42.7562 mL |
| 5 mM | 0.342 mL | 1.7102 mL | 3.4205 mL | 6.841 mL | 8.5512 mL |
| 10 mM | 0.171 mL | 0.8551 mL | 1.7102 mL | 3.4205 mL | 4.2756 mL |
| 50 mM | 0.0342 mL | 0.171 mL | 0.342 mL | 0.6841 mL | 0.8551 mL |
| 100 mM | 0.0171 mL | 0.0855 mL | 0.171 mL | 0.342 mL | 0.4276 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hydroxypropyl tetrahydropyrantriol
Catalog No.:BCX1142
CAS No.:439685-79-7
- Presenegenin
Catalog No.:BCX1141
CAS No.:2163-40-8
- Ligustrosidic acid
Catalog No.:BCX1140
CAS No.:96382-89-7
- 3α-Hydroxymogrol
Catalog No.:BCX1139
CAS No.:1343402-73-2
- N-methyltyramine
Catalog No.:BCX1138
CAS No.:370-98-9
- Ganosporeric acid A
Catalog No.:BCX1137
CAS No.:135357-25-4
- Avenanthramide A
Catalog No.:BCX1136
CAS No.:108605-70-5
- Avenanthramide B
Catalog No.:BCX1135
CAS No.:108605-69-2
- 3-Feruloyl-4-caffeoylquinic acid
Catalog No.:BCX1134
CAS No.:96990-65-7
- β-Sitosteryl acetate
Catalog No.:BCX1133
CAS No.:915-05-9
- Tetraacetylphytosphingosine
Catalog No.:BCX1132
CAS No.:13018-48-9
- L-Guluronic Acid Sodium Salt
Catalog No.:BCX1131
CAS No.:15769-56-9
- Reptoside
Catalog No.:BCX1144
CAS No.:53839-03-5
- Jaligonic acid
Catalog No.:BCX1145
CAS No.:51776-39-7
- 24(28)-Dehydroergosterol
Catalog No.:BCX1146
CAS No.:29560-24-5
- Eicosapentaenoic acid
Catalog No.:BCX1147
CAS No.:10417-94-4
- Toralactone
Catalog No.:BCX1148
CAS No.:41743-74-2
- Rubropunctatin
Catalog No.:BCX1149
CAS No.:514-67-0
- Methyl brevifolincarboxylate
Catalog No.:BCX1150
CAS No.:154702-76-8
- Butyl neochlorogenate
Catalog No.:BCX1151
CAS No.:409361-64-4
- Butyl chlorogenate
Catalog No.:BCX1152
CAS No.:132741-56-1
- Spicatine A
Catalog No.:BCX1153
CAS No.:124256-81-1
- Aljesaconitine B
Catalog No.:BCX1154
CAS No.:101247-24-9
- Pyroside
Catalog No.:BCX1155
CAS No.:10338-88-2
Chemical and biochemical characterization of Ipomoea aquatica: genoprotective potential and inhibitory mechanism of its phytochemicals against alpha-amylase and alpha-glucosidase.[Pubmed:38192648]
Front Nutr. 2023 Dec 21;10:1304903.
Ipomea aquatica, also known as water spinach, is an aquatic non-conventional leafy vegetable and is considered a healthy and seasonal delicacy in ethnic food culture. The study revealed the presence of rich chemical and biochemical composition in I. aquatica and antioxidant activities. Moreover, the plant extracts demonstrated significant DNA damage prevention activity against UV/H(2)O(2)-induced oxidative damage. High-resolution mass spectrometric analysis by UPLC-qTOF-MS/MS resulted in the identification of over 65 different compounds and 36 important secondary metabolites. Most of the compounds identified represented polyphenolic compounds, viz. polyphenol glycosides and phenolic acids, followed by alkaloids and terpenoids. A UPLC-DAD method was developed and quantified for 10 different polyphenolic compounds. Out of all the metabolites examined, a significant number of compounds were reported to have various bioactive properties, including antibacterial, antiviral, antitumor, hepatoprotection, and anti-depressant effects. The plant extracts were found to contain various compounds, including Euphornin, lucidenic acid, and myricitin glycosides, which possess significant medicinal value. Metabolite analysis utilizing GC-MS revealed the presence of various fatty acids, amino acids, sugars, and organic acids. The analysis revealed the presence of essential unsaturated fatty acids such as alpha-linolenic acid as well as beneficial substances such as squalene., The evaluation of glycemic control activity was carried out by comprehending the inhibitory potential of alpha-amylase and alpha-glucosidase, outlining the kinetics of the inhibition process. The inhibitory activities were compared to those of acarbose and revealed stronger inhibition of alpha-glucosidase as compared to alpha-amylase. Furthermore, the mechanism of inhibition was determined using in silico analysis, which involved molecular docking and molecular dynamic simulation of the identified IA phytochemicals complexed with the hydrolase enzymes. The study generates convincing evidence that dietary intake of I. aquatica provides a positive influence on glycemic control along with various health-protective and health-promoting benefits.
Photo-induced scandium-catalyzed biomimetic skeleton conversion of lathyrane to naturally rare eupholathone Euphorbia diterpenes.[Pubmed:37752884]
Chem Commun (Camb). 2023 Oct 12;59(82):12290-12293.
The naturally scarce eupholathone-type Euphornin E (1) was efficiently prepared from abundant lathyrane-type Euphorbia factor L(1)via a visible-light-induced Sc(OTf)(3)-catalyzed tandem process. Eupholathones 2 and 3 were also smoothly obtained by changing the reaction solvent. This route provides a convenient method for easily constructing scarce eupholathone- from lathyrane-type Euphorbia diterpenes, and confirms the biogenetic relationship between them from a chemical standpoint. Notably, compound 1 exhibited good anti-HIV activity.
Euphornin L promotes lipid clearance by dual regulation of LDLR and PCSK9.[Pubmed:34650629]
Exp Ther Med. 2021 Dec;22(6):1381.
Our previous study identified Euphornin L as an active lipid-lowering compound in high-fat diet-fed Golden Syrian hamsters. The aim of the present study was to investigate the mechanisms underlying the lipid-lowering effects of Euphornin L. Euphornin L in HepG2 cells was assessed via DiI-LDL update assays and found to increase LDL-update and LDLR protein levels. RNA interference assays demonstrated that its LDL-update effects were LDLR-dependent. Dual luciferase reporter and mRNA stability assays revealed that Euphornin L had little effect on LDLR mRNA transcription but lengthened the half-life of LDLR mRNA by activating ERK protein in cells. Euphornin L decreased the secretion of PCSK9 protein and alleviated PCSK9-mediated LDLR protein degradation. In vivo experiments in hamsters, which were treated with Euphornin L (30 mg/kg/day) for 3 weeks, confirmed these findings. LDLR protein levels in liver tissue were upregulated, while PCSK9 protein levels in serum were downregulated. Altogether, the present study demonstrated that Euphornin L increased LDLR protein levels by dual regulation of LDLR mRNA and PCSK9 protein, and represented an active compound for lipid-lowering drug development.
Euphornin reduces proliferation of human cervical adenocarcinoma HeLa cells through induction of apoptosis and G2/M cell cycle arrest.[Pubmed:30100745]
Onco Targets Ther. 2018 Jul 27;11:4395-4405.
BACKGROUND: The plant Euphorbia helioscopia L. has been used in traditional Chinese medicine for treating various disorders such as tuberculosis and edema. The aim of this study was to investigate the effect of Euphornin, a bioactive compound isolated from E. helioscopia, on proliferation of human cervical adenocarcinoma HeLa cells by analyzing cell viability, rate of apoptosis, and cell cycle progression. MATERIALS AND METHODS: The sulforhodamine B assay was used to study the effect of Euphornin on the proliferation of HeLa cells. Morphological changes to cell nuclei were identified after Hoechst 33342 staining. Mitochondrial membrane depolarization (MMP) was analyzed after staining with JC-1 dye. The influence of Euphornin on the apoptosis rate was analyzed by Annexin V/propidium iodide double staining. Fluorescence-activated cell sorting was applied to investigate the influence of Euphornin on cell cycle progression. Proteins were obtained from HeLa cells and analyzed by Western blots. RESULTS: A cell viability assay showed that Euphornin inhibited proliferation of HeLa cells in a dose-dependent and time-dependent manner. Euphornin also induced apoptosis in a concentration-dependent manner, with the rates of apoptosis ranging from 25.3% to 52.6%. A high concentration of Euphornin was found to block HeLa cells at the G2/M stage. A Western blot analysis suggested that Euphornin might exhibit antitumor activity by inducing apoptosis. Euphornin treatment altered the ratio of Bax/Bcl-2 in HeLa cells, which led to the release of cytochrome complex. The levels of cleaved caspase-3, caspase-8, caspase-9, and caspase-10 were also markedly increased by Euphornin treatment. Analysis of cell cycles indicated that Euphornin induced cell cycle arrest by increasing the level of the phospho-CDK1 (Tyr15) protein. The various assays demonstrated that Euphornin treatment resulted in a significant suppression of cell growth accompanied by G2/M cell cycle arrest and increased rate of apoptosis via mitochondrial and caspase pathways. CONCLUSION: Our findings suggest that Euphornin has the potential to be used as a cancer therapeutic agent against human cervical adenocarcinoma.
Jatrophanes as promising multidrug resistance modulators: Advances of structure-activity relationships.[Pubmed:29447979]
Fitoterapia. 2018 Jun;127:138-145.
The phytochemical study of Euphorbia helioscopia afforded Euphornin (1) in a large amount. Alkaline hydrolysis of 1 using potassium carbonate yielded the main product monodeacetylEuphornin (2), whose structural modification at 14-OH gave rise to 21 acylated derivatives euphornoate A-U (3-23). Thus, a mini compound library of jatrophanes was established to screen for MDR modulators. Biological studies clearly demonstrated the effect of C-14 pattern modification in MDR reversal activity and several compounds with RF values over 300 fold at 20 muM (6, 16, 20, 22, 23) were thought to be promising MDR modulators. The SARs are discussed, which reveal that introduction of an alkyl acyl group bearing 4 carbons at C-14 or an aryl acyl group with electron donating groups is favorable for the activity.
Secoheliosphanes A and B and Secoheliospholane A, Three Diterpenoids with Unusual seco-Jatrophane and seco-Jatropholane Skeletons from Euphorbia helioscopia.[Pubmed:29188714]
J Org Chem. 2018 Jan 5;83(1):167-173.
Secoheliosphanes A (1) and B (2) and secoheliospholane A (3), possessing an unusual 7,8-seco-jatrophane skeleton and an unprecedented 9,10-seco-7,10-epoxyjatropholane skeleton, respectively, were isolated from the whole plants of Euphorbia helioscopia, along with two biogenetically precursors, a new jatrophane diterpene, 2-epi-Euphornin I (4) and a known jatrophane diterpene, euphoscopin A (5). Structures of 1-4 including absolute configurations were elucidated on the basis of spectroscopic data, X-ray crystallography, and chemical conversion. Compounds 1 and 2 were prepared from 4 and 5, respectively, confirming their structural assignments. Notably, 1 and 2 presented the first examples of seco-jatrophane-type diterpenoids and 3 featured a novel 5/6/7/7-fused tetracyclic ring skeleton. Among them, compound 2 showed modest activity against HSV-1 with IC(50) value of 6.41 muM.
[Study on chemical constituents of Euphorbia helioscopia and their antitumor activities].[Pubmed:24417144]
Zhong Yao Cai. 2013 Jul;36(7):1092-6.
OBJECTIVE: To investigate the chemical constituents of Euphorbia helioscopia and their antitumor activities. METHODS: Normal phase silica gel, RP-18 silica gel and Sephadex LH-20 column chromatographies combined with recrystallization were used to isolate and purify the constituents. Their structures were identifided by spectroscopic methods, including 1H-NMR, 13C-NMR, ESI-MS and EI-MS. And the antitumor activities of some of chemical constituents in vitro were detected by sulphorhodamine B protein staining. RESULTS: Nine compounds were isolated and their structures were identified as euphohelioscopin A (1), euphoscopin (2), 9, 19-cyclolanost-23E-ene-3, 25-diol (3), euphoscopin C (4), Euphornin A (5), euphoheliosnoid A (6), ent-kaurane-3-oxo-16beta, 17-diol (7), 9, 19-cyclolanost-25-ene-3beta, 22-diol (8) and helioscopinolide A(9) Compound 9 showed effect on inhibiting the cell proliferations of MCF-7 cell line. CONCLUSION: Compounds 3, 7, 8 and 9 are obtained from this plant for the first time, and compound 9 shows the potential antitumor activity.
Analysis of euphornin in Euphorbia helioscopia L. and its cytotoxicity to mice lung adenocarcinoma cells (LA795).[Pubmed:21958384]
Nat Prod Res. 2012;26(22):2112-6.
Euphorbia helioscopia L. has been used as a herbal remedy for cancer in mainland China. Euphornin is one of the main bioactive constituents with the maximal content of Euphorbia helioscopia L. A reversed-phase high-performance liquid chromatography method with evaporative light scattering detection (ELSD) was developed for the analysis of Euphornin for better quality control of E. helioscopia L. A good calibration curve in double logarithmic coordinator for Euphornin was obtained. The validation study showed high recoveries (>97.0%) and low coefficient of variation (<3.0%). The use of the method on different Euphornin extract samples confirmed its effectiveness. It was shown that ELSD was an effective detection method for the analysis of the non-volatile diterpenes from plants used in traditional Chinese medicine. The evaluation of the cytotoxicity of Euphornin to mice lung adenocarcinoma cells (LA795) suggested that Euphornin was one of the constituents of E. helioscopia L. responsible for the cytotoxicity against carcinoma cells.
Cytotoxic macrocyclic diterpenoids from Euphorbia helioscopia.[Pubmed:19099222]
Arch Pharm Res. 2008 Dec;31(12):1547-51.
A new cytotoxic macrocyclic diterpenoid, Euphornin L (1), together with seven known analogues were isolated from the plant Euphorbia helioscopia L. The structure of 1 was elucidated by spectral data and X-ray crystallographic analysis. Euphornin L (1) and euphoscopin F (2) exhibited significant cytotoxicity against HL-60 cell lines with IC(50) values of 2.7 and 9.0 microM, respectively. The 13C-NMR data of euphoscopin F (2), epieuphoscopin B (3), euphoscopin B (5), and euphoscopin C (6) were also reported for the first time.
Discovery of a new series of jatrophane and lathyrane diterpenes as potent and specific P-glycoprotein modulators.[Pubmed:18452010]
Org Biomol Chem. 2008 May 21;6(10):1756-62.
A new series of diterpenes, the jatrophanes euphoscopin M (1), euphoscopin N (2) and Euphornin L (3), and the lathyrane euphohelioscopin C (7) were isolated from plants of Euphorbia helioscopia L., together with four other known analogues, euphoscopin C (4), Euphornin (5), epieuphoscopin B (6) and euphohelioscopin A (8). The new compound stereostructures were elucidated by NMR analysis and computational data. The resulting isolated diterpenes were found to be potent inhibitors of P-glycoprotein (ABCB1), while showing an absence of significant activity against BCRP (ABCG2), despite the high substrate overlapping of these transporters, thus including them in the third-generation class of specific multidrug transporter modulators.
Cytotoxic diterpenoids from Euphorbia helioscopia.[Pubmed:18321057]
J Nat Prod. 2008 May;71(5):873-6.
Four new jatrophane-type diterpenoids (1-4), together with 16 known related compounds, were isolated from the Chinese medicinal plant Euphorbia helioscopia. The structures of 1-4 were determined on the basis of spectroscopic and chemical methods. Cytotoxicity of the isolated compounds against HeLa and MDA-MB-231 cells was evaluated, with only helioscopinolide A (5) and Euphornin (3a) being active.


