N-methyltyramineCAS# 370-98-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 370-98-9 | SDF | Download SDF |
| PubChem ID | 9727.0 | Appearance | Powder |
| Formula | C9H13NO | M.Wt | 151.21 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
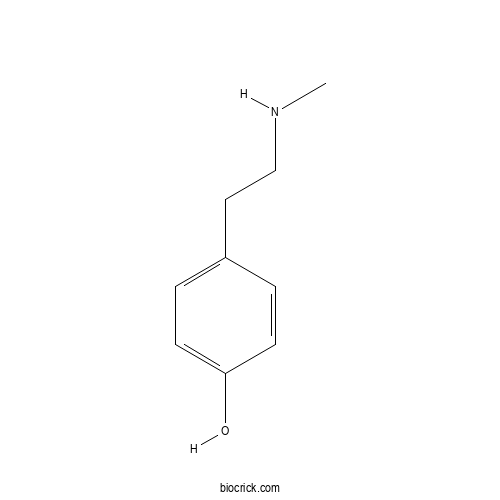
| Chemical Name | 4-[2-(methylamino)ethyl]phenol | ||
| SMILES | CNCCC1=CC=C(C=C1)O | ||
| Standard InChIKey | AXVZFRBSCNEKPQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H13NO/c1-10-7-6-8-2-4-9(11)5-3-8/h2-5,10-11H,6-7H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

N-methyltyramine Dilution Calculator

N-methyltyramine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.6133 mL | 33.0666 mL | 66.1332 mL | 132.2664 mL | 165.333 mL |
| 5 mM | 1.3227 mL | 6.6133 mL | 13.2266 mL | 26.4533 mL | 33.0666 mL |
| 10 mM | 0.6613 mL | 3.3067 mL | 6.6133 mL | 13.2266 mL | 16.5333 mL |
| 50 mM | 0.1323 mL | 0.6613 mL | 1.3227 mL | 2.6453 mL | 3.3067 mL |
| 100 mM | 0.0661 mL | 0.3307 mL | 0.6613 mL | 1.3227 mL | 1.6533 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ganosporeric acid A
Catalog No.:BCX1137
CAS No.:135357-25-4
- Avenanthramide A
Catalog No.:BCX1136
CAS No.:108605-70-5
- Avenanthramide B
Catalog No.:BCX1135
CAS No.:108605-69-2
- 3-Feruloyl-4-caffeoylquinic acid
Catalog No.:BCX1134
CAS No.:96990-65-7
- β-Sitosteryl acetate
Catalog No.:BCX1133
CAS No.:915-05-9
- Tetraacetylphytosphingosine
Catalog No.:BCX1132
CAS No.:13018-48-9
- L-Guluronic Acid Sodium Salt
Catalog No.:BCX1131
CAS No.:15769-56-9
- Ostruthine
Catalog No.:BCX1130
CAS No.:148-83-4
- Agigenin
Catalog No.:BCX1129
CAS No.:55332-76-8
- Salidroside pentaacetate
Catalog No.:BCX1128
CAS No.:39032-08-1
- 2,3,4,6-tetraacetate Salidroside
Catalog No.:BCX1127
CAS No.:28251-63-0
- (-)-Epitaxifolin
Catalog No.:BCX1126
CAS No.:114761-89-6
- 3α-Hydroxymogrol
Catalog No.:BCX1139
CAS No.:1343402-73-2
- Ligustrosidic acid
Catalog No.:BCX1140
CAS No.:96382-89-7
- Presenegenin
Catalog No.:BCX1141
CAS No.:2163-40-8
- Hydroxypropyl tetrahydropyrantriol
Catalog No.:BCX1142
CAS No.:439685-79-7
- Euphornin
Catalog No.:BCX1143
CAS No.:80454-47-3
- Reptoside
Catalog No.:BCX1144
CAS No.:53839-03-5
- Jaligonic acid
Catalog No.:BCX1145
CAS No.:51776-39-7
- 24(28)-Dehydroergosterol
Catalog No.:BCX1146
CAS No.:29560-24-5
- Eicosapentaenoic acid
Catalog No.:BCX1147
CAS No.:10417-94-4
- Toralactone
Catalog No.:BCX1148
CAS No.:41743-74-2
- Rubropunctatin
Catalog No.:BCX1149
CAS No.:514-67-0
- Methyl brevifolincarboxylate
Catalog No.:BCX1150
CAS No.:154702-76-8
Simultaneous mass spectrometric quantification of trace amines, their precursors and metabolites.[Pubmed:38583227]
J Chromatogr B Analyt Technol Biomed Life Sci. 2024 May 1;1238:124098.
OBJECTIVES: Trace amines are powerful neuromodulators influencing the release and reuptake of catecholamines. These low concentrated endogenous amines impact mood, cognition, and hormone regulation. Dysregulation of trace amines have been associated with a variety of diseases, such as schizophrenia, Parkinson's disease, migraine, depression and more. Succesfull simultaneous quantification of trace amines, their precursors and metabolites would benefit both research and patient care. Since these compounds have various functional groups and are present in biological matrices with large concentration difference, their simultaneous quantification is an analytical challenge. Our goal was to develop a highly sensitive LC-MS/MS assay to simultaneously quantify trace amines, their precursors and metabolites in plasma. METHODS: Our method is based on a simple two-step in-matrix derivatization protocol: propionic anhydride (PA) and 3-Ethyl-1-[3-(dimethylamino)propyl]carbodiimide (EDC) in combination with 2,2,2-trifluoroethylamine (TFEA) followed by online solid phase extraction combined with LC-MS/MS. Fifteen metabolites can be measured simultaneously, three precursors, eight trace amines and four metabolites. Validation of this method was performed according to international validation guidelines. The pre-analytical stability of trace amines was assessed. RESULTS: This novel method was successful in quantifying trace amines, their precursors, and metabolites in plasma. Using just 50 microl human plasma, we were able to accomplish limit of quantification for 2-phenylethylamine and N-methyl-phenylethylamine of 0.2 nmol/L and 0.1 nmol/L for tyramine and N-methyltyramine. Inter-and intra-assay imprecision was < 15 % for all analytes. Stability assessment showed susceptibility of certain trace amines e.g. 2-phenylethylamine and N-methyl-phenylethylamine to enzymatic degradation in plasma. The addition of the monoamine oxidase inhibitor pargyline to plasma prevented this enzymatic degradation. CONCLUSIONS: We developed a novel LC-MS/MS method that1) uses a new double derivatization technique, 2) is automated with online SPE, 3) uses far less sample volume then previous methods and 4) detects more components in the same sample (eight trace amines, three precursors, and four metabolites) with high specificity and selectivity. Furthermore, addition of MAO A/B inhibitor prevents degradation and guarantees more accurate quantification of trace amines.
Physical, Nutritional, and Bioactive Properties of Mandacaru Cladode Flour (Cereus jamacaru DC.): An Unconventional Food Plant from the Semi-Arid Brazilian Northeast.[Pubmed:36496622]
Foods. 2022 Nov 26;11(23):3814.
In this study, we evaluated the physical, nutritional, and bioactive properties of mandacaru cladode flour (Cereus jamacaru DC.). The granulometric profile revealed particles with non-uniform geometry, flakiness, a rectangular tendency, and a non-homogeneous surface, with particle sizes ranging from 20 to 60 microm. The flour presented low water activity (0.423), a moisture content of 8.24 g/100 g, high ash (2.82 g/100 g), protein (5.18 g/100 g), and total carbohydrate contents (74.48 g/100 g), and low lipid contents (1.88 g/100 g). Mandacaru flour is an excellent source of insoluble dietary fiber (48.08 g/100 g), calcium (76.33%), magnesium (15.21%), and potassium (5.94%). Notably, (1)H NMR analysis revealed the presence of N-methyltyramine. Using HPLC chromatography, glucose was identified as the predominant sugar (1.33 g/100 g), followed by four organic acids, especially malic acid (9.41 g/100 g) and citric acid (3.96 g/100 g). Eighteen phenolic compounds were detected, with relevant amounts of kaempferol (99.40 mg/100 g), myricetin (72.30 mg/100 g), and resveratrol (17.84 mg/100 g). The total phenolic compounds and flavonoids were 1285.47 mg GAE/100 g and 15.19 mg CE/100 g, respectively. The mean in vitro antioxidant activity values were higher using the FRAP method (249.45 micromol Trolox TEAC/100 g) compared to the ABTS(*+) method (0.39 micromol Trolox TEAC/g). Finally, the ascorbic acid had a content of 35.22 mg/100 g. The results demonstrate the value of mandacaru as a little-explored species and an excellent matrix for the development of flours presenting good nutritional value and bioactive constituents with excellent antioxidant potential.
UPLC-Q-TOF-MS/MS analysis on the chemical composition of malts under different germination cycles and prepared with different processing methods.[Pubmed:36179899]
Fitoterapia. 2023 Mar;165:105313.
OBJECTIVE: To investigate changes in the chemical composition of malts under different germination cycles and prepared with different processing methods, thus providing a reference for the clinical application of malt in disease treatment. METHODS: Nine malt samples were analyzed by ultra-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF-MS/MS), and the MS fragmentation pathway of 4 compounds (including hordenine, gramine, N-methyltyramine and catechin) were also analyzed. RESULTS: By database comparison and literature search, we detected 31 compounds in raw barley and 33 compounds in both raw malt and roasted malt. Nonetheless, the most of these 33 compounds were detected higher contents in raw malt than in roasted malt. Besides, we detected 15 compounds in brown malt. At Day1 of germination, 31 compounds were detected in malt, without two alkaloids (representative: hordenine). At Day2-5, 33 compounds were detected, with different contents as shown by the peak area comparison; hordenine had a gradually increasing abundance; and nearly one third of the chemical components in barley increased gradually, one third decreased gradually, and one third tended to be stable. CONCLUSION: Malts under different germination cycles and prepared with different processing methods have varying active ingredients, and especially brown malt exhibits a serious loss of compounds. The tight association between the chemical composition and clinical application of malt offers a basis to the clinically scientific and reasonable selection of Chinese medicinal materials for treatment purposes.
Multiple Direct Effects of the Dietary Protoalkaloid N-Methyltyramine in Human Adipocytes.[Pubmed:35956295]
Nutrients. 2022 Jul 29;14(15):3118.
Dietary amines have been the subject of a novel interest in nutrition since the discovery of trace amine-associated receptors (TAARs), especially TAAR-1, which recognizes tyramine, phenethylamine, tryptamine, octopamine, N-methyltyramine (NMT), synephrine, amphetamine and related derivatives. Alongside the psychostimulant properties of TAAR-1 ligands, it is their ephedrine-like action on weight loss that drives their current consumption via dietary supplements advertised for 'fat-burning' properties. Among these trace amines, tyramine has recently been described, at high doses, to exhibit an antilipolytic action and activation of glucose transport in human adipocytes, i.e., effects that are facilitating lipid storage rather than mobilization. Because of its close structural similarity to tyramine, NMT actions on human adipocytes therefore must to be reevaluated. To this aim, we studied the lipolytic and antilipolytic properties of NMT together with its interplay with insulin stimulation of glucose transport along with amine oxidase activities in adipose cells obtained from women undergoing abdominal surgery. NMT activated 2-deoxyglucose uptake when incubated with freshly isolated adipocytes at 0.01-1 mM, reaching one-third of the maximal stimulation by insulin. However, when combined with insulin, NMT limited by half the action of the lipogenic hormone on glucose transport. The NMT-induced stimulation of hexose uptake was sensitive to inhibitors of monoamine oxidases (MAO) and of semicarbazide-sensitive amine oxidase (SSAO), as was the case for tyramine and benzylamine. All three amines inhibited isoprenaline-induced lipolysis to a greater extent than insulin, while they were poorly lipolytic on their own. All three amines-but not isoprenaline-interacted with MAO or SSAO. Due to these multiple effects on human adipocytes, NMT cannot be considered as a direct lipolytic agent, potentially able to improve lipid mobilization and fat oxidation in consumers of NMT-containing dietary supplements.
Effects of Hippeastrum reticulatum on memory, spatial learning and object recognition in a scopolamine-induced animal model of Alzheimer's disease.[Pubmed:33170051]
Pharm Biol. 2020 Dec;58(1):1098-1104.
CONTEXT: The methanol extracts from Hippeastrum reticulatum (L'Her.) Herb. (Amaryllidaceae) (HR) display acetylcholinesterase inhibitory (AChEI) activity. OBJECTIVE: AChEI of alkaloids isolated from HR bulbs and the ameliorating effects of the alkaloid fraction (AHR) on memory and cognitive dysfunction in scopolamine-treated mice were investigated. MATERIALS AND METHODS: Alkaloids were isolated by column chromatography and identified by spectroscopy. AChEI was evaluated using the modified Ellman's method. Sixty Swiss male mice were randomly divided into six groups, received samples for 15 days. Normal group received saline, scopolamine-treated group scopolamine (1.5 mg/kg, intraperitoneal injection). Test groups received AHR (5, 10 and 15 mg/kg, per os) and positive control group donepezil (5 mg/kg, per os), administered 1 h before the test, scopolamine was injected 30 min prior to testing. The cognitive-enhancing activity of AHR against scopolamine-induced memory impairments was investigated using Y-maze, the novel object recognition test (NORT) and the Morris water maze (MWM) test. RESULTS: Seven alkaloids were isolated for the first time from the genus Hippeastrum: trans-dihydronarciclasine (1), N-chloromethylnarcissidinium (2), narciprimin (3), narciclasine-4-O-beta-d-xylopyranoside (4), N-methyltyramine (5), 3beta,11alpha-dihydroxy-1,2-dehydrocrinane (6) and brunsvigine (7); three are new compounds (2, 5, 6). Among them, 2-3 and 5-6 showed AChEI in vitro with IC(50) values of 29.1, 46.4, 70.1 and 104.5 microg/mL, respectively. The anti-AChEI of 2, 5 and 6 are reported for the first time. In in vivo test, AHR (15 mg/kg) significantly increased in spontaneous alternation performance in the Y-maze test (p < 0.01), it significantly increased the time spent exploring the novel object (p < 0.05) comparison with scopolamine-treated group. The administration of AHR at doses 10 and 15 mg/kg significantly decreased escapes latency and swimming distance to the platform on day 6 compared to these in day 1 (p < 0.01 and p < 0.05, respectively). CONCLUSIONS: AHR could be a potential candidate of future trials for treatment of memory and cognitive dysfunction in Alzheimer's disease.
Analysis of bitter orange dietary supplements for natural and synthetic phenethylamines by LC-MS/MS.[Pubmed:32497396]
Drug Test Anal. 2020 Sep;12(9):1241-1251.
Citrus aurantium, commonly known as bitter orange, is a popular dietary supplement ingredient sold worldwide. Bitter orange supplements are sold primarily as weight management and sports performance products and have gained popularity after Ephedra products were banned from the US market. Supplements containing synephrine are reported to exhibit adverse cardiovascular effects especially in the presence of caffeine. In this study, an LC-MS/MS method was established to quantify five natural amines (synephrine, octopamine, tyramine, N-methyltyramine, and hordenine) and four synthetic phenethylamines (phenylephrine, methylsynephrine, etilefrine, and isopropyloctopamine) in dietary supplements sold in the US. The method was validated and found to have acceptable performance to accurately measure analytes in complex botanical products. The average recoveries from a blank matrix were 88-125% with an RSD of 0.5-7.0%. Fifty-nine products labeled to contain bitter orange peel, extract, or its amines were purchased and their amine content was measured. Several products were found to contain higher amounts of amines than that expected from a typical bitter orange extract. Of the 23 products that made label claims for synephrine, only 5 products (22%) were within 80-120% of labeled synephrine content. The presence of synthetic amines, methylsynephrine (up to 240 mg/daily serving), and isopropyloctopamine (up to 76 mg/daily serving), whose effects in humans are not known, were detected in six products and one product, respectively. While the use of methylsynephrine and isopropyloctopamine are not permitted in dietary supplements, hordenine, N-methyltyramine, and octopamine are currently listed on the FDA's Dietary Supplement Ingredient Advisory List.
New insights in the allelopathic traits of different barley genotypes: Middle Eastern and Tibetan wild-relative accessions vs. cultivated modern barley.[Pubmed:32324789]
PLoS One. 2020 Apr 23;15(4):e0231976.
The two alkaloids gramine and hordenine have been known for playing a role in the allelopathic ability in barley (Hordeum vulgare L.). These allelochemicals can be both found in leaves and roots in some barley cultivars whereas in others one seems to exclude the other. In this study eighteen accessions of barley from the Middle-East area, one accession from Tibet and the modern spring cultivar Barke, already used as parental donor in a nested associated mapping (NAM) population, were screened for their gramine, hordenine and N-methyltyramine (the direct precursor of hordenine) content in leaves, roots and exudates. Moreover, the toxicity of the three allelochemicals on root growth inhibition on lettuce (Lactuca sativa L.) was evaluated. Results of this study showed the preferential production of gramine and hordenine in leaves and roots, respectively, in the nineteen barley accessions. On the other hand, in the modern barley cultivar Barke, the highest content of hordenine in roots and the general lack of gramine suggests a favored biosynthesis of the former. Gramine was not detected in the root exudates. In additions, different metabolomic profiles were observed in wild relatives compared to modern barley genotypes. The results also showed the phytotoxic effects of the three compounds on root growth of lettuce seedlings, with a reduction in root length and an increase of root surface area and diameter. In conclusion, this study highlighted the impact of the domestication effects on the production and distribution of the two allelopathic alkaloids gramine and hordenine in barley.
Absorption, Biokinetics, and Metabolism of the Dopamine D2 Receptor Agonist Hordenine (N,N-Dimethyltyramine) after Beer Consumption in Humans.[Pubmed:31984737]
J Agric Food Chem. 2020 Feb 19;68(7):1998-2006.
Hordenine, a natural constituent of germinated barley, is a biased agonist of the dopamine D2 receptor. This pilot study investigated the biokinetics of hordenine and its metabolites in four volunteers consuming beer equal to 0.075 mg hordenine/kg body weight. A new ultrahigh-performance liquid chromatography method coupled to electrospray ionization tandem mass spectrometry (UHPLC-ESI-MS/MS) method determined maximum plasma concentrations of 12.0-17.3 nM free hordenine after 0-60 min. Hordenine phase-II metabolism was first dominated by sulfation, but later by glucuronidation. The elimination half-lives in plasma were 52.7-66.4 min for free hordenine and about 60/80 min longer for hordenine sulfate and hordenine glucuronide. Urinary excretion peaked 2-3.5 h after consumption and accumulated to 3.78 mumol within 24 h, corresponding to 9.9% of the ingested dose. The observed hordenine levels in plasma seem too low to provoke direct interaction with the dopamine D2 receptor related to food reward, but synergistic or additive effects with alcohol or N-methyltyramine may occur.
Effects of the amino acid derivatives, beta-hydroxy-beta-methylbutyrate, taurine, and N-methyltyramine, on triacylglycerol breakdown in fat cells.[Pubmed:30919256]
J Physiol Biochem. 2019 Aug;75(3):263-273.
Various amino acid (AA) metabolites are used as supplements to facilitate metabolic control and enhance responsiveness of insulin-sensitive tissues. beta-hydroxy-beta-methylbutyrate (HMB) is a leucine metabolite proposed to prevent muscle wasting and to mitigate insulin resistance. Taurine, commonly added to energizing drinks, is a metabolite of methionine and cysteine present in bile juice, and proposed to be involved in lipid digestion and to be pro-lipolytic in adipocytes. N-methyltyramine (NMT) is a phenylalanine metabolite found in orange juices at 0.1-3 ppm while its effects on lipid mobilization remain controversial. Here, the putative lipolytic effects of these AA metabolites were studied and it was tested whether they could enhance insulin antilipolytic response in adipocytes. Release of glycerol and non-esterified fatty acids (NEFAs) was measured after a 2-h incubation of adipocytes obtained from control and diet-induced obese mice or from obese patients. In mouse, none of the tested AA derivatives was lipolytic from 1 muM to 1 mM. These compounds did not improve insulin antilipolytic effect or isoprenaline lipolytic action, except for 1 mM NMT that impaired triacylglycerol breakdown in obese mice. In human adipocytes, HMB and taurine were not lipolytic, while NMT weakly activated glycerol and NEFA release at 1 mM. However, 100 muM NMT impaired isoprenaline-stimulated lipolysis in a manner that was hardly added to insulin antilipolytic effect. Since none of these AA derivatives acutely helped or replaced insulin antilipolytic effect in adipocytes, the present in vitro observations do not support their proposed insulin-sensitizing properties. Moreover, NMT, HMB, and taurine were not notably lipolytic.
Effect of N-methyltyramine on the regulation of adrenergic receptors via enzymatic epinephrine synthesis for the treatment of gastrointestinal disorders.[Pubmed:30841454]
Biomed Pharmacother. 2019 Mar;111:1393-1398.
BACKGROUND: Citri Reticulatae Pericarpium (CRP), Aurantii Fructus Immaturus (AFI) and Aurantii Fructus (AF) are all important Citrus species used in traditional Chinese medicines (TCMs) for the treatment of gastrointestinal disorders. Although they have been used since ancient times and are still in use today, the mechanistic basis for their regulation of adrenergic receptors (ARs) is still not clear. PURPOSE: In this study, we aimed to determine the active components and mechanisms of action of CRP, AFI and AF in treating gastrointestinal disorders related to ARs. METHODS: First, the phenethylamine alkaloid components of CRP, AFI and AF were identified and compared across 30 samples of three Citrus species by UPLC-Q/TOF-MS in combination with content difference analysis. Second, the effect of the main active alkaloid component on AR-based gastrointestinal disorders was investigated by an in vivo small intestinal propulsive test and an in vitro relaxing small intestinal smooth muscle activity test. The mechanism of AR regulation of the active alkaloid was further studied by evaluating its effect on relaxing small intestinal smooth muscle in the presence of an inhibitor. Lastly, the enzymes, which played an important role in epinephrine synthesis and AR regulation, were detected by immunohistochemistry. RESULTS: Three phenethylamine AR regulators (N-methyltyramine, synephrine and hordenine) in CRP, AFI and AF were characterized. It was found that N-methyltyramine could relax mouse small intestinal smooth muscle and inhibit small intestinal propulsion. The effect of N-methyltyramine on relaxing small intestinal smooth muscle could be inhibited by a-methyl-l-tyrosine. The enzymes related epinephrine synthesis and AR function were found in the mouse small intestine. The biotransformation process that converts N-methyltyramine to epinephrine was determined. CONCLUSION: The treatment of gastrointestinal disorders of CRP, AFI and AF is associated with their alkaloid component N-methyltyramine via the regulation of ARs, and the mechanism is considered to be the biotransformation of N-methyltyramine to epinephrine by serial synthase, which takes place at the nerves cells in small intestine.
Monitoring of the dopamine D2 receptor agonists hordenine and N-methyltyramine during the brewing process and in commercial beer samples.[Pubmed:30409657]
Food Chem. 2019 Mar 15;276:745-753.
The phenethylamine alkaloid hordenine, present in germinated barley, was identified recently as a functionally selective dopamine D2 receptor agonist contributing potentially to the rewarding effects of drinking beer. Here, it was shown that the hordenine precursor N-methyltyramine binds with a similar affinity to the dopamine D2 receptor as hordenine (K(i) 31.3 microM) showing also selectivity towards the G protein-mediated pathway over the beta-arrestin pathway. Using a newly developed UHPLC-ESI-MS/MS method to monitor beer production, we demonstrated that hordenine and N-methyltyramine were released continuously from barley malt during mashing and were stable during fermentation and conditioning. The amounts released from different base malt types were in a similar range but tended to be higher from caramel malts. Hordenine and N-methyltyramine concentrations in 24 types of beer varied between 1.05-6.32 and 0.59-4.61 mg/L, respectively. Thus, the human uptake of the alkaloids during beer consumption is in the low milligram range.
Detection and quantification of phenethylamines in sports dietary supplements by NMR approach.[Pubmed:29413984]
J Pharm Biomed Anal. 2018 Mar 20;151:347-355.
Phenethylamines (PEAs) are popular substances found in weight-loss and sports nutrition supplements. They are generally pharmacologically active and primarily affect the sympathetic nervous system. Many PEAs are synthetic chemicals and are on the prohibited list of the World Anti-Doping Agency. In this study, nuclear magnetic resonance (NMR) spectroscopy was applied to detect and identify the presence of PEAs in sports dietary supplements without the need for chromatographic separation or pre-knowledge on formulation. Eight PEAs, viz. phenethylamine, synephrine, oxilofrine, hordenine, beta-methylphenethylamine, N-methyltyramine, octopamine and deterenol, were identified from 32 dietary supplements sold in the US market. Furthermore, a quantitative NMR method was developed and validated for simultaneous determination of the concentrations of the PEAs. The study demonstrated that NMR could be a potential tool to monitor and detect PEAs or other ingredients in dietary supplements.


