AgigeninCAS# 55332-76-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 55332-76-8 | SDF | Download SDF |
| PubChem ID | 44566818.0 | Appearance | Powder |
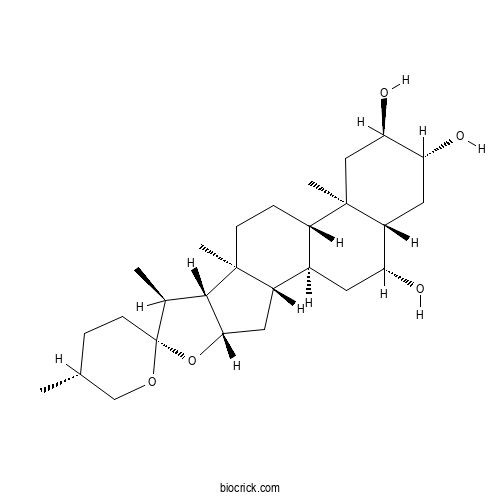
| Formula | C27H44O5 | M.Wt | 448.64 |
| Type of Compound | Steroids and their saponins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,2S,4S,5'R,6R,7S,8R,9S,12S,13R,15R,16R,18S,19R)-5',7,9,13-tetramethylspiro[5-oxapentacyclo[10.8.0.02,9.04,8.013,18]icosane-6,2'-oxane]-15,16,19-triol | ||
| SMILES | CC1CCC2(C(C3C(O2)CC4C3(CCC5C4CC(C6C5(CC(C(C6)O)O)C)O)C)C)OC1 | ||
| Standard InChIKey | FYRLHXNMINIDCB-LEGLVIAUSA-N | ||
| Standard InChI | InChI=1S/C27H44O5/c1-14-5-8-27(31-13-14)15(2)24-23(32-27)11-18-16-9-20(28)19-10-21(29)22(30)12-26(19,4)17(16)6-7-25(18,24)3/h14-24,28-30H,5-13H2,1-4H3/t14-,15+,16-,17+,18+,19-,20-,21-,22-,23+,24+,25+,26-,27-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Agigenin Dilution Calculator

Agigenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.229 mL | 11.1448 mL | 22.2896 mL | 44.5792 mL | 55.724 mL |
| 5 mM | 0.4458 mL | 2.229 mL | 4.4579 mL | 8.9158 mL | 11.1448 mL |
| 10 mM | 0.2229 mL | 1.1145 mL | 2.229 mL | 4.4579 mL | 5.5724 mL |
| 50 mM | 0.0446 mL | 0.2229 mL | 0.4458 mL | 0.8916 mL | 1.1145 mL |
| 100 mM | 0.0223 mL | 0.1114 mL | 0.2229 mL | 0.4458 mL | 0.5572 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Salidroside pentaacetate
Catalog No.:BCX1128
CAS No.:39032-08-1
- 2,3,4,6-tetraacetate Salidroside
Catalog No.:BCX1127
CAS No.:28251-63-0
- (-)-Epitaxifolin
Catalog No.:BCX1126
CAS No.:114761-89-6
- Zymosterol
Catalog No.:BCX1125
CAS No.:128-33-6
- Jaligonic acid B
Catalog No.:BCX1124
CAS No.:2375176-78-4
- Monensin B
Catalog No.:BCX1123
CAS No.:30485-16-6
- Beta-Tomatine
Catalog No.:BCX1122
CAS No.:17406-46-1
- Orchioside B
Catalog No.:BCX1121
CAS No.:851780-22-8
- Abyssinone II
Catalog No.:BCX1120
CAS No.:77263-08-2
- Sphingomyelin
Catalog No.:BCX1119
CAS No.:6254-89-3
- 22,23-Dihydroergosterol
Catalog No.:BCX1118
CAS No.:516-79-0
- Inflacoumarin A
Catalog No.:BCX1117
CAS No.:158446-33-4
- Ostruthine
Catalog No.:BCX1130
CAS No.:148-83-4
- L-Guluronic Acid Sodium Salt
Catalog No.:BCX1131
CAS No.:15769-56-9
- Tetraacetylphytosphingosine
Catalog No.:BCX1132
CAS No.:13018-48-9
- β-Sitosteryl acetate
Catalog No.:BCX1133
CAS No.:915-05-9
- 3-Feruloyl-4-caffeoylquinic acid
Catalog No.:BCX1134
CAS No.:96990-65-7
- Avenanthramide B
Catalog No.:BCX1135
CAS No.:108605-69-2
- Avenanthramide A
Catalog No.:BCX1136
CAS No.:108605-70-5
- Ganosporeric acid A
Catalog No.:BCX1137
CAS No.:135357-25-4
- N-methyltyramine
Catalog No.:BCX1138
CAS No.:370-98-9
- 3α-Hydroxymogrol
Catalog No.:BCX1139
CAS No.:1343402-73-2
- Ligustrosidic acid
Catalog No.:BCX1140
CAS No.:96382-89-7
- Presenegenin
Catalog No.:BCX1141
CAS No.:2163-40-8
Revisiting the South Indian Traditional Plants against Several Targets of SARS-CoV-2 - An in silico Approach.[Pubmed:36588334]
Curr Comput Aided Drug Des. 2023;19(3):202-233.
BACKGROUND: The south Indian Telugu states will celebrate a new year called 'Ugadi' which is a south Indian traditional festival. The ingredients used in ugadi pachadi have often also been used in food as well as traditional Ayurveda and Siddha medicinal preparations. Coronaviruses (CoVs) are a diverse family of enveloped positive-sense single-stranded RNA viruses which can infect humans and have the potential to cause large-scale outbreaks. OBJECTIVE: Considering the benefits of ugadi pachadi, we investigated the binding modes of various phytochemical constituents reported from its ingredients against five targets of SARS-CoV-2. METHODS: Flexible-ligand docking simulations were achieved through AutoDock version 1.5.6. Following 50ns of molecular dynamics simulation using GROMACS 2018.1 software and binding free energy (DeltaG(bind)) of the protein-ligand complexes were calculated using the g_mmpbsa tool. ADME prediction was done using Qikprop of Schrodinger. RESULTS: From the molecular docking and MM/PBSA results compound Eriodictin exhibited the highest binding energy when complexed with nucleocapsid N protein (6M3M) (-6.8 kcal/mol, - 82.46 kJ/mol), bound SARS-CoV-2-hACE2 complex (6M0J) (-7.4 kcal/mol, -71.10 kJ/mol) and Mpro (6XR3) (-8.6 kcal/mol, -140.21 kJ/mol). Van der Waal and electrostatic energy terms highly favored total free energy binding. CONCLUSION: The compounds Eriodictin, Vitexin, Cycloart-3, 24, 27-triol, Agigenin, Mangiferin, Mangiferolic acid, Schaftoside, 27-Hydroxymangiferonic acid, Quercetin, Azadirachtol, Cubebin, Isomangiferin, Isoquercitrin, Malicarpin, Orientin and procyanidin dimer exhibited satisfactory binding energy values when compared with standard molecules. The further iterative optimization of high-ranked compounds following validation by in vitro and in vivo techniques assists in discovering therapeutic anti-SARS-CoV-2 molecules.
Spirostanol Saponins from Flowers of Allium Porrum and Related Compounds Indicating Cytotoxic Activity and Affecting Nitric Oxide Production Inhibitory Effect in Peritoneal Macrophages.[Pubmed:34770942]
Molecules. 2021 Oct 29;26(21):6533.
Saponins, a diverse group of natural compounds, offer an interesting pool of derivatives with biomedical application. In this study, three structurally related spirostanol saponins were isolated and identified from the leek flowers of Allium porrum L. (garden leek). Two of them were identical with the already known leek plant constituents: aginoside (1) and 6-deoxyaginoside (2). The third one was identified as new component of A. porrum; however, it was found identical with yayoisaponin A (3) obtained earlier from a mutant of elephant garlic Allium ampeloprasun L. It is a derivative of the aginoside (1) with additional glucose in its glycosidic chain, identified by MS and NMR analysis as (2alpha, 3beta, 6beta, 25R)-2,6-dihydroxyspirostan-3-yl beta-D-glucopyranosyl-(1 --> 3)-beta-D-glucopranosyl-(1 --> 2)-[beta-D-xylopyranosyl-(1 --> 3)]-beta-D-glucopyranosyl]-(1 --> 4)-beta-D-galactopyranoside, previously reported also under the name alliporin. The leek native saponins were tested together with other known and structurally related saponins (tomatonin and digitonin) and with their related aglycones (Agigenin and diosgenin) for in vitro cytotoxicity and for effects on NO production in mouse peritoneal cells. The highest inhibitory effects were exhibited by 6-deoxyaginoside. The obtained toxicity data, however, closely correlated with the suppression of NO production. Therefore, an unambiguous linking of obtained bioactivities of saponins with their expected immunobiological properties remained uncertain.
Antifungal saponins from bulbs of garlic, Allium sativum L. var. Voghiera.[Pubmed:22513009]
Phytochemistry. 2012 Jun;78:126-34.
A bioassay-guided phytochemical analysis of the polar extract from the bulbs of garlic, Allium sativum L., var. Voghiera, typical of Voghiera, Ferrara (Italy), allowed the isolation of ten furostanol saponins; voghieroside A1/A2 and voghieroside B1/B2, based on the rare agapanthagenin aglycone; voghieroside C1/C2, based on Agigenin aglycone; and voghieroside D1/D2 and E1/E2, based on gitogenin aglycone. In addition, we found two known spirostanol saponins, Agigenin 3-O-trisaccharide and gitogenin 3-O-tetrasaccharide. The chemical structures of the isolated compounds were established through a combination of extensive nuclear magnetic resonance, mass spectrometry and chemical analyses. High concentrations of two eugenol diglycosides were also found for the first time in Allium spp. The isolated compounds were evaluated for their antimicrobial activity towards two fungal species, the air-borne pathogen Botrytis cinerea and the antagonistic fungus Trichoderma harzianum.
Anti-HBV active flavone glucosides from Euphorbia humifusa Willd.[Pubmed:20450964]
Fitoterapia. 2010 Oct;81(7):799-802.
Thirteen flavone glucosides from the herb of Euphorbia humifusa were isolated and elucidated. Among them, five compounds including apigenin-7-O-beta-D-glucopyranoside (2), apigenin-7-O-(6''-O-galloyl)-beta-D-glucopyranoside (3), luteolin-7-O-beta-D-glucopyranoside (7), luteolin-7-O-(6''-O-trans-feruloyl)-beta-D-glucopyranoside (8) and luteolin-7-O-(6''-O-coumaroyl)-beta-D-glucopyranoside (9) showed anti-HBV activity in vitro. The structure-activity relationship showed that the parent structure was closely relevant to the anti-HBV activity of these compounds (Agigenin>luteolin>quercetin). It was found that the number of glucoside in the structure may significantly influence their activities (flavone monoglucoside>flavone diglucoside) and cytotoxicity (flavone>flavone monoglucoside>flavone diglucoside). In addition, the substitution of acyl group on glucoside may be important to keep the anti-HBV activities of these compounds (galloyl>feruloyl>coumaroyl).
Porrigenins A and B, novel cytotoxic and antiproliferative sapogenins isolated from Allium porrum.[Pubmed:9358643]
J Nat Prod. 1997 Oct;60(10):1003-7.
Four new sapogenins, porrigenins A (2a) and B (3a), identified as (25R)-5 alpha-spirostan-2 beta,3 beta,6 beta-triol and (25R)-2-oxo-5 alpha-spirostan-3 beta,6 beta-diol, respectively, and neoporrigenins A (2b) and B (3b) were also isolated from Allium porrum. In addition, the known Agigenin (1a) and its 25S epimer, neoAgigenin (1b), were also identified. Their structure elucidation was provided by comprehensive spectroscopic analyses. Compounds 1a, 2a, and 3a exhibited cytotoxicity and high antiproliferative activity on four different tumor cell lines in vitro.
[Experimental study of the anabolic activity of 6-ketoderivatives of certain natural sapogenins].[Pubmed:1028596]
Farmakol Toksikol. 1976 Sep-Oct;39(5):631-5.
It is shown that 6-ketoderivatives of natural sapogenins, viz. Agigenin, diosgenin and alliogenin, display the anabolic activity and do not manifest any androgenic properties. The compoud IV/(25 R)-5alpha-spirostan-2alpha, 3beta, 5alpha-triol-6-OH/produces an accelerated gain of weight in rats, and also an increase in the weight of the liver, heart, kidneys, musculus tibiliasis anterior and augments the total amount of protein therein. All of the above-mentioned changes become more pronounced with the study substance introduced to young animals. Castration of sexually immature rats greatly mitigates the anabolic effect of the compound IV.


