RehmapicrogeninCAS# 135447-39-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

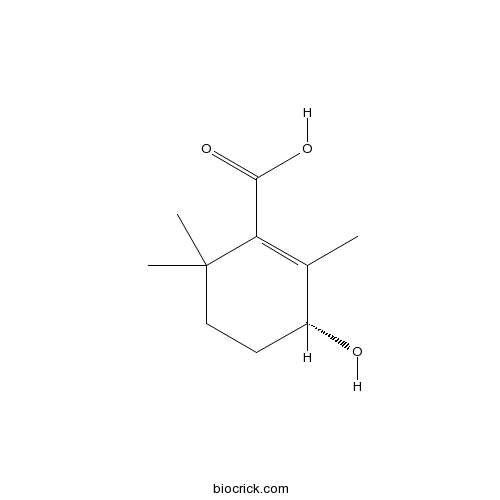
| Cas No. | 135447-39-1 | SDF | Download SDF |
| PubChem ID | 15693863 | Appearance | White powder |
| Formula | C10H16O3 | M.Wt | 184.232 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3R)-3-hydroxy-2,6,6-trimethylcyclohexene-1-carboxylic acid | ||
| SMILES | CC1=C(C(CCC1O)(C)C)C(=O)O | ||
| Standard InChIKey | IJTFWVKHFTZVSR-SSDOTTSWSA-N | ||
| Standard InChI | InChI=1S/C10H16O3/c1-6-7(11)4-5-10(2,3)8(6)9(12)13/h7,11H,4-5H2,1-3H3,(H,12,13)/t7-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Rehmapicrogenin Dilution Calculator

Rehmapicrogenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4279 mL | 27.1397 mL | 54.2794 mL | 108.5588 mL | 135.6985 mL |
| 5 mM | 1.0856 mL | 5.4279 mL | 10.8559 mL | 21.7118 mL | 27.1397 mL |
| 10 mM | 0.5428 mL | 2.714 mL | 5.4279 mL | 10.8559 mL | 13.5698 mL |
| 50 mM | 0.1086 mL | 0.5428 mL | 1.0856 mL | 2.1712 mL | 2.714 mL |
| 100 mM | 0.0543 mL | 0.2714 mL | 0.5428 mL | 1.0856 mL | 1.357 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7-O-Prenylscopoletin
Catalog No.:BCN3547
CAS No.:13544-37-1
- JW 480
Catalog No.:BCC6142
CAS No.:1354359-53-7
- trans-Ned 19
Catalog No.:BCC7825
CAS No.:1354235-96-3
- ACT 335827
Catalog No.:BCC6346
CAS No.:1354039-86-3
- KH CB19
Catalog No.:BCC6135
CAS No.:1354037-26-5
- Bullatine A
Catalog No.:BCN2374
CAS No.:1354-84-3
- CX-6258 hydrochloride hydrate
Catalog No.:BCC1505
CAS No.:1353858-99-7
- 8-Prenyldaidzein
Catalog No.:BCN4711
CAS No.:135384-00-8
- 12alpha-Methoxygrandiflorenic acid
Catalog No.:BCN7771
CAS No.:135383-94-7
- 4'-Hydroxyisojasminin
Catalog No.:BCN7383
CAS No.:135378-09-5
- Isojasminin
Catalog No.:BCN7492
CAS No.:135378-08-4
- 5,7,3',4'-Tetrahydroxy-3-methoxy-8,5'-diprenylflavone
Catalog No.:BCN6848
CAS No.:1353676-65-9
- Strontium Ranelate
Catalog No.:BCC3858
CAS No.:135459-87-9
- Araliadiol
Catalog No.:BCC8835
CAS No.:1354638-93-9
- Cabazitaxel intermediate
Catalog No.:BCN7432
CAS No.:1354900-65-4
- CYM 50260
Catalog No.:BCC6259
CAS No.:1355026-60-6
- ML 190
Catalog No.:BCC6308
CAS No.:1355244-02-8
- 2,3-Dihydro-12,13-dihydroxyeuparin
Catalog No.:BCN7189
CAS No.:135531-75-8
- Mutant IDH1-IN-1
Catalog No.:BCC6403
CAS No.:1355326-21-4
- AGI-5198
Catalog No.:BCC2293
CAS No.:1355326-35-0
- Cudraxanthone L
Catalog No.:BCN6187
CAS No.:135541-40-1
- Schisanlignone A
Catalog No.:BCN3628
CAS No.:135557-67-4
- Nav1.7 inhibitor
Catalog No.:BCC4191
CAS No.:1355631-24-1
- Pneumocandin B0
Catalog No.:BCC5436
CAS No.:135575-42-7
Bioassay-guided isolation of anti-inflammatory components from the root of Rehmannia glutinosa and its underlying mechanism via inhibition of iNOS pathway.[Pubmed:23034094]
J Ethnopharmacol. 2012 Oct 11;143(3):867-75.
ETHNOPHARMACOLOGICAL RELEVANCE: The root of Rehmannia glutinosa (RR) is commonly used to reduce inflammation in various traditional Chinese herbal formulae; however, little is known regarding its active component(s). AIM OF STUDY: The objective of the present study was to examine the active component(s) responsible for the anti-inflammatory activity of RR via anti-nitric oxide production assay-guided fractionation; and the underlying anti-inflammatory mechanism of action of such component(s) was further investigated. MATERIALS AND METHODS: Anti-nitric oxide (NO) activities with lipopolysaccharides (LPS)-stimulated RAW264.7 murine macrophages was used as screening platform. Gene, protein and inflammatory mediators' expression were also studied using real-time PCR, western blotting and ELISA, respectively. RESULTS: Using anti-NO assay-guided fractionation, sub-fraction C3 (from 31.25 to 62.5 mug/ml, p=0.001 to 0.01) possessed 100-fold more potent anti-inflammatory effect than that of the aqueous extract of RR. Characterization of C3 showed that the anti-inflammatory effect could be partly due to the presence of Rehmapicrogenin, which could significantly inhibit NO production (p<0.001). C3 was further demonstrated in blocking inflammation by inhibiting gene (p<0.001) and protein expression of inducible NO synthase (iNOS) dose-dependently. Besides, C3 also significantly inhibited the production of prostaglandin E(2) (p<0.001 to 0.01), IL-6 (p<0.001 to 0.05) and COX-2 (p<0.05). CONCLUSIONS: Rehmapicrogenin was, for the first time, shown to possess nitric oxide inhibitory activities. Bioassay-guided fractionation demonstrated that Rehmapicrogenin-containing subfraction C3 exhibited potent anti-inflammatory effect by inhibiting iNOS, COX-2 and IL-6, while Rehmapicrogenin was only partially responsible for the anti-inflammatory effect of RR.


