Mutant IDH1-IN-1CAS# 1355326-21-4 |
- PSI-7977
Catalog No.:BCC1871
CAS No.:1190307-88-0
- Trovafloxacin mesylate
Catalog No.:BCC3931
CAS No.:147059-75-4
- Capecitabine
Catalog No.:BCN2168
CAS No.:154361-50-9
- Viomycin
Catalog No.:BCC3930
CAS No.:32988-50-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1355326-21-4 | SDF | Download SDF |
| PubChem ID | 89696514 | Appearance | Powder |
| Formula | C30H31FN4O2 | M.Wt | 498.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 45 mg/mL (90.25 mM) *"≥" means soluble, but saturation unknown. | ||
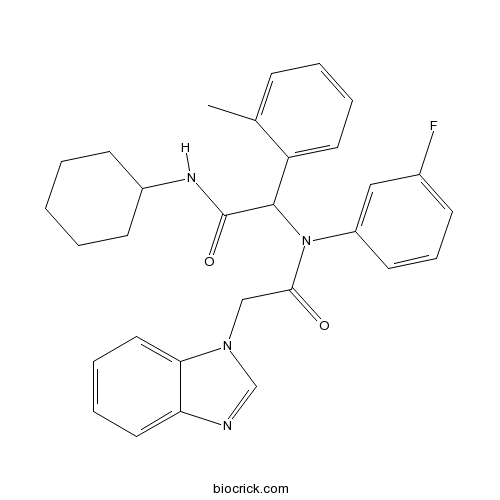
| Chemical Name | 2-(N-[2-(benzimidazol-1-yl)acetyl]-3-fluoroanilino)-N-cyclohexyl-2-(2-methylphenyl)acetamide | ||
| SMILES | CC1=CC=CC=C1C(C(=O)NC2CCCCC2)N(C3=CC(=CC=C3)F)C(=O)CN4C=NC5=CC=CC=C54 | ||
| Standard InChIKey | DXYIOARJXVTYJW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C30H31FN4O2/c1-21-10-5-6-15-25(21)29(30(37)33-23-12-3-2-4-13-23)35(24-14-9-11-22(31)18-24)28(36)19-34-20-32-26-16-7-8-17-27(26)34/h5-11,14-18,20,23,29H,2-4,12-13,19H2,1H3,(H,33,37) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Mutant IDH1-IN-1 Dilution Calculator

Mutant IDH1-IN-1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0057 mL | 10.0283 mL | 20.0566 mL | 40.1131 mL | 50.1414 mL |
| 5 mM | 0.4011 mL | 2.0057 mL | 4.0113 mL | 8.0226 mL | 10.0283 mL |
| 10 mM | 0.2006 mL | 1.0028 mL | 2.0057 mL | 4.0113 mL | 5.0141 mL |
| 50 mM | 0.0401 mL | 0.2006 mL | 0.4011 mL | 0.8023 mL | 1.0028 mL |
| 100 mM | 0.0201 mL | 0.1003 mL | 0.2006 mL | 0.4011 mL | 0.5014 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Mutant IDH1-IN-1 is a mutant-selective IDH1 inhibitor with with IC50s of 4, 42, 80 and 143 nM against mutant IDH1 R132C/R132C, IDH1 R132H/R132H, IDH1 R132H/WT and wild type IDH1, respectively.
References:
[1]. Deng G, et al. Selective inhibition of mutant isocitrate dehydrogenase 1 (IDH1) via disruption of a metal binding network by an allosteric small molecule. J Biol Chem. 2015 Jan 9;290(2):762-74.
- 2,3-Dihydro-12,13-dihydroxyeuparin
Catalog No.:BCN7189
CAS No.:135531-75-8
- ML 190
Catalog No.:BCC6308
CAS No.:1355244-02-8
- CYM 50260
Catalog No.:BCC6259
CAS No.:1355026-60-6
- Cabazitaxel intermediate
Catalog No.:BCN7432
CAS No.:1354900-65-4
- Araliadiol
Catalog No.:BCC8835
CAS No.:1354638-93-9
- Strontium Ranelate
Catalog No.:BCC3858
CAS No.:135459-87-9
- Rehmapicrogenin
Catalog No.:BCN8507
CAS No.:135447-39-1
- 7-O-Prenylscopoletin
Catalog No.:BCN3547
CAS No.:13544-37-1
- JW 480
Catalog No.:BCC6142
CAS No.:1354359-53-7
- trans-Ned 19
Catalog No.:BCC7825
CAS No.:1354235-96-3
- ACT 335827
Catalog No.:BCC6346
CAS No.:1354039-86-3
- KH CB19
Catalog No.:BCC6135
CAS No.:1354037-26-5
- AGI-5198
Catalog No.:BCC2293
CAS No.:1355326-35-0
- Cudraxanthone L
Catalog No.:BCN6187
CAS No.:135541-40-1
- Schisanlignone A
Catalog No.:BCN3628
CAS No.:135557-67-4
- Nav1.7 inhibitor
Catalog No.:BCC4191
CAS No.:1355631-24-1
- Pneumocandin B0
Catalog No.:BCC5436
CAS No.:135575-42-7
- Parsonsianidine
Catalog No.:BCN2109
CAS No.:135601-81-9
- Fmoc-D-His(Trt)-OH
Catalog No.:BCC3504
CAS No.:135610-90-1
- Coccineone B
Catalog No.:BCN6464
CAS No.:135626-13-0
- CP 80633
Catalog No.:BCC7463
CAS No.:135637-46-6
- 17-Methylparsonsianidine
Catalog No.:BCN2093
CAS No.:135637-68-2
- Fmoc-Cha-OH
Catalog No.:BCC3160
CAS No.:135673-97-1
- Narchinol B
Catalog No.:BCN7796
CAS No.:1356822-09-7
Durable Control of Metastatic AKT1-Mutant WHO Grade 1 Meningothelial Meningioma by the AKT Inhibitor, AZD5363.[Pubmed:28376212]
J Natl Cancer Inst. 2017 Mar 1;109(3):1-4.
High-throughput analyses have revealed the presence of activating mutations in the AKT1 gene in a subpopulation of meningiomas. We report a female patient with multiple intracranial tumor manifestations and histologically verified meningotheliomatous meningioma in the lung. The tumor was continuously growing at multiple sites despite six surgical resections, radiotherapy, and two lines of systemic therapy. Following detection of an AKT1E17K mutation in three independent tumor samples by sequencing, treatment with AZD5363, a selective AKT inhibitor, was initiated. Ex vivo cultured meningioma cells exhibited sensitivity to the drug as shown by pAKT accumulation on immunoblots. Treatment with AZD5363 resulted, for the first time, in stable disease and minor radiographic response. The patient has been on that treatment for more than one year with ongoing clinical and radiographic response. This is the first report of an AKT1-mutant meningioma responding to AKT inhibition, suggesting that molecular screening may result in clinical benefit.
Co-activation of STAT3 and YES-Associated Protein 1 (YAP1) Pathway in EGFR-Mutant NSCLC.[Pubmed:28376152]
J Natl Cancer Inst. 2017 Sep 1;109(9). pii: 3076962.
Background: The efficacy of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in EGFR-mutant non-small cell lung cancer (NSCLC) is limited by adaptive activation of cell survival signals. We hypothesized that both signal transducer and activator of transcription 3 (STAT3) and Src-YES-associated protein 1 (YAP1) signaling are dually activated during EGFR TKI treatment to limit therapeutic response. Methods: We used MTT and clonogenic assays, immunoblotting, and quantitative polymerase chain reaction to evaluate the efficacy of EGFR TKI alone and in combination with STAT3 and Src inhibition in three EGFR-mutant NSCLC cell lines. The Chou-Talalay method was used for the quantitative determination of drug interaction. We examined tumor growth inhibition in one EGFR-mutant NSCLC xenograft model (n = 4 mice per group). STAT3 and YAP1 expression was evaluated in tumors from 119 EGFR-mutant NSCLC patients (64 in an initial cohort and 55 in a validation cohort) by quantitative polymerase chain reaction. Kaplan-Meier and Cox regression analyses were used to assess the correlation between survival and gene expression. All statistical tests were two-sided. Results: We discovered that lung cancer cells survive initial EGFR inhibitor treatment through activation of not only STAT3 but also Src-YAP1 signaling. Cotargeting EGFR, STAT3, and Src was synergistic in two EGFR-mutant NSCLC cell lines with a combination index of 0.59 (95% confidence interval [CI] = 0.54 to 0.63) for the PC-9 and 0.59 (95% CI = 0.54 to 0.63) for the H1975 cell line. High expression of STAT3 or YAP1 predicted worse progression-free survival (hazard ratio [HR] = 3.02, 95% CI = 1.54 to 5.93, P = .001, and HR = 2.57, 95% CI = 1.30 to 5.09, P = .007, respectively) in an initial cohort of 64 EGFR-mutant NSCLC patients treated with firstline EGFR TKIs. Similar results were observed in a validation cohort. Conclusions: Our study uncovers a coordinated signaling network centered on both STAT3 and Src-YAP signaling that limits targeted therapy response in lung cancer and identifies an unforeseen rational upfront polytherapy strategy to minimize residual disease and enhance clinical outcomes.
Development and Genetic Characterization of A Novel Herbicide (Imazethapyr) Tolerant Mutant in Rice (Oryza sativa L.).[Pubmed:28378144]
Rice (N Y). 2017 Dec;10(1):10.
BACKGROUND: Increased water and labour scarcity in major rice growing areas warrants a shift towards direct seeded rice cultivation under which management of weeds is a major issue. Use of broad spectrum non-selective herbicides is an efficient means to manage weeds. Availability of rice genotypes with complete tolerance against broad-spectrum non-selective herbicides is a pre-requisite for advocating use of such herbicides. In the present study, we developed an EMS induced rice mutant, 'HTM-N22', exhibiting tolerance to a broad spectrum herbicide, 'Imazethapyr', and identified the mutations imparting tolerance to the herbicide. RESULTS: We identified a stable and true breeding rice mutant, HTM-N22 (HTM), tolerant to herbicide, Imazethapyr, from an EMS-mutagenized population of approximately 100,000 M2 plants of an upland rice variety, Nagina 22 (N22). Analysis of inheritance of herbicide tolerance in a cross between Pusa 1656-10-61/HTM showed that this trait is governed by a single dominant gene. To identify the causal gene for Imazethapyr tolerance, bulked segregant analysis (BSA) was followed using microsatellite markers flanking the three putative candidate genes viz., an Acetolactate Synthase (ALS) on chromosome 6 and two Acetohydroxy Acid Synthase (AHAS) genes, one on chromosomes 2 and another on chromosome 4. RM 6844 on chromosome 2 located 0.16 Mbp upstream of AHAS (LOC_Os02g30630) was found to co-segregate with herbicide tolerance. Cloning and sequencing of AHAS (LOC_Os02g30630) from the wild type, N22 and the mutant HTM and their comparison with reference Nipponbare sequence revealed several Single Nucleotide Polymorphisms (SNPs) in the mutant, of which eight resulted in non-synonymous mutations. Three of the eight amino acid substitutions were identical to Nipponbare and hence were not considered as causal changes. Of the five putative candidate SNPs, four were novel (at positions 30, 50, 81 and 152) while the remaining one, S627D was a previously reported mutant, known to result in Imidazolinone tolerance in rice. Of the novel ones, G152E was found to alter the hydrophobicty and abolish an N myristoylation site in the HTM compared to the WT, from reference based modeling and motif prediction studies. CONCLUSIONS: A novel mutant tolerant to the herbicide "Imazethapyr" was developed and characterized for genetic, sequence and protein level variations. This is a HTM in rice without any IPR (Intellectual Property Rights) infringements and hence can be used in rice breeding as a novel genetic stock by the public funded organizations in the country and elsewhere.
Characterization of Brucella abortus mutant strain Delta22915, a potential vaccine candidate.[Pubmed:28376905]
Vet Res. 2017 Apr 4;48(1):17.
Brucellosis, caused by Brucella spp., is an important zoonosis worldwide. Vaccination is an effective strategy for protection against Brucella infection in livestock in developing countries and in wildlife in developed countries. However, current vaccine strains including S19 and RB51 are pathogenic to humans and pregnant animals, limiting their use. In this study, we constructed the Brucella abortus (B. abortus) S2308 mutant strain Delta22915, in which the putative lytic transglycosylase gene BAB_RS22915 was deleted. The biological properties of mutant strain Delta22915 were characterized and protection of mice against virulent S2308 challenge was evaluated. The mutant strain Delta22915 showed reduced survival within RAW264.7 cells and survival in vivo in mice. In addition, the mutant strain Delta22915 failed to escape fusion with lysosomes within host cells, and caused no observable pathological damage. RNA-seq analysis indicated that four genes associated with amino acid/nucleotide transport and metabolism were significantly upregulated in mutant strain Delta22915. Furthermore, inoculation of 22915 at 10(5) colony forming units induced effective host immune responses and long-term protection of BALB/c mice. Therefore, mutant strain 22915 could be used as a novel vaccine candidate in the future to protect animals against B. abortus infection.


