Strontium RanelateCAS# 135459-87-9 |
- Mibefradil
Catalog No.:BCC1748
CAS No.:116644-53-2
- Mibefradil dihydrochloride
Catalog No.:BCC1749
CAS No.:116666-63-8
- Cilnidipine
Catalog No.:BCC1083
CAS No.:132203-70-4
- NNC 55-0396
Catalog No.:BCC1803
CAS No.:357400-13-6
- NP118809
Catalog No.:BCC1807
CAS No.:41332-24-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 135459-87-9 | SDF | Download SDF |
| PubChem ID | 6918182 | Appearance | Powder |
| Formula | C12H6N2O8SSr2 | M.Wt | 513.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Distrontium renelate; S12911 | ||
| Solubility | DMSO : < 1 mg/mL (insoluble or slightly soluble) | ||
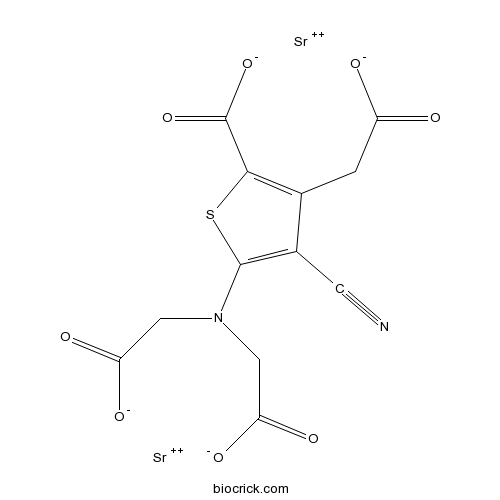
| Chemical Name | distrontium;5-[bis(carboxylatomethyl)amino]-3-(carboxylatomethyl)-4-cyanothiophene-2-carboxylate | ||
| SMILES | C(C1=C(SC(=C1C#N)N(CC(=O)[O-])CC(=O)[O-])C(=O)[O-])C(=O)[O-].[Sr+2].[Sr+2] | ||
| Standard InChIKey | XXUZFRDUEGQHOV-UHFFFAOYSA-J | ||
| Standard InChI | InChI=1S/C12H10N2O8S.2Sr/c13-2-6-5(1-7(15)16)10(12(21)22)23-11(6)14(3-8(17)18)4-9(19)20;;/h1,3-4H2,(H,15,16)(H,17,18)(H,19,20)(H,21,22);;/q;2*+2/p-4 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Strontium ranelate(S12911) stimulates the calcium sensing receptors (CaSR) and leads to the differentiation of pre-osteoblast to osteoblast which increases the bone formation.
IC50 value:
Target: CaSR
Strontium Ranelate is a bone metabolism modulator that inhibits bone resorption while maintaining bone formation. Strontium Ranelate acts by increasing bone formation and decreasing bone resorption, thus rebalancing bone turnover in favour of bone formation, an effect that results in increased bone mass and strength. Commonly used as an antiosteoporotic. Strontium Ranelate has shown efficacy in preventing early postmenopausal bone loss and reducing the risk of hip fracture in women with postmenopausal osteoporosis. References: | |||||

Strontium Ranelate Dilution Calculator

Strontium Ranelate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9475 mL | 9.7373 mL | 19.4746 mL | 38.9492 mL | 48.6864 mL |
| 5 mM | 0.3895 mL | 1.9475 mL | 3.8949 mL | 7.7898 mL | 9.7373 mL |
| 10 mM | 0.1947 mL | 0.9737 mL | 1.9475 mL | 3.8949 mL | 4.8686 mL |
| 50 mM | 0.0389 mL | 0.1947 mL | 0.3895 mL | 0.779 mL | 0.9737 mL |
| 100 mM | 0.0195 mL | 0.0974 mL | 0.1947 mL | 0.3895 mL | 0.4869 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Strontium Ranelate is a bone metabolism modulator that inhibits bone resorption while maintaining bone formation. Commonly used as an antiosteoporotic.
- Rehmapicrogenin
Catalog No.:BCN8507
CAS No.:135447-39-1
- 7-O-Prenylscopoletin
Catalog No.:BCN3547
CAS No.:13544-37-1
- JW 480
Catalog No.:BCC6142
CAS No.:1354359-53-7
- trans-Ned 19
Catalog No.:BCC7825
CAS No.:1354235-96-3
- ACT 335827
Catalog No.:BCC6346
CAS No.:1354039-86-3
- KH CB19
Catalog No.:BCC6135
CAS No.:1354037-26-5
- Bullatine A
Catalog No.:BCN2374
CAS No.:1354-84-3
- CX-6258 hydrochloride hydrate
Catalog No.:BCC1505
CAS No.:1353858-99-7
- 8-Prenyldaidzein
Catalog No.:BCN4711
CAS No.:135384-00-8
- 12alpha-Methoxygrandiflorenic acid
Catalog No.:BCN7771
CAS No.:135383-94-7
- 4'-Hydroxyisojasminin
Catalog No.:BCN7383
CAS No.:135378-09-5
- Isojasminin
Catalog No.:BCN7492
CAS No.:135378-08-4
- Araliadiol
Catalog No.:BCC8835
CAS No.:1354638-93-9
- Cabazitaxel intermediate
Catalog No.:BCN7432
CAS No.:1354900-65-4
- CYM 50260
Catalog No.:BCC6259
CAS No.:1355026-60-6
- ML 190
Catalog No.:BCC6308
CAS No.:1355244-02-8
- 2,3-Dihydro-12,13-dihydroxyeuparin
Catalog No.:BCN7189
CAS No.:135531-75-8
- Mutant IDH1-IN-1
Catalog No.:BCC6403
CAS No.:1355326-21-4
- AGI-5198
Catalog No.:BCC2293
CAS No.:1355326-35-0
- Cudraxanthone L
Catalog No.:BCN6187
CAS No.:135541-40-1
- Schisanlignone A
Catalog No.:BCN3628
CAS No.:135557-67-4
- Nav1.7 inhibitor
Catalog No.:BCC4191
CAS No.:1355631-24-1
- Pneumocandin B0
Catalog No.:BCC5436
CAS No.:135575-42-7
- Parsonsianidine
Catalog No.:BCN2109
CAS No.:135601-81-9
[Effects of strontium ranelate on the expression of BMP-2 in the mid-palatal suture of rats during rapid maxillary expansion].[Pubmed:28275783]
Shanghai Kou Qiang Yi Xue. 2016 Dec;25(6):647-651.
PURPOSE: To investigate the effects of Strontium Ranelate on the expression of BMP-2 during rapid maxillary expansion. METHODS: Thirty-six male 6-week- old male Wistar rats were selected. They were randomly divided into 3 groups. Group A was designed as a control group. An expanded application producing 100 g force was fixed between the first and the second molar on both sides of the rats in group B and group C. 600 mg/kg Strontium Ranelate was given to the rats in group C daily via an orogastric route, while the equal normal saline was given to the rats in group B. Then the rats were sacrificed on day 4, 7 and 10. The expression of BMP-2 which is a sign of bone formation was detected with immunohistochemical staining and analyzed with Image-pro plus 5.0. SPSS19.0 software package was used for statistical analysis. RESULTS: The expression of BMP-2 in the midpalatal suture was significantly greater in group B than that in group A on day 4 (P<0.05); however, there was no significant difference between group A and group B on day 7 and 10 (P>0.05). The expression of BMP-2 in the midpalatal suture in group C was significantly greater than that in the other two groups at each time point (P<0.05). CONCLUSIONS: Strontium Ranelate promotes the expression time and quantity of BMP-2 in the mid-palatal suture of rats during rapid maxillary expansion and may accelerate bone formation during rapid maxillary expansion.
[Effects of strontium ranelate on the rats' palatal suture after rapid maxillary expansion].[Pubmed:28317347]
Hua Xi Kou Qiang Yi Xue Za Zhi. 2016 Aug 1;34(4):336-340.
OBJECTIVE: This study investigated the effects of Strontium Ranelate (SrR) on the rats' palatal suture after rapid maxillary expansion (RME). METHODS: Thirty-six male 6-week-old Wistar rats were randomly divided into three groups: control group (A), expansion only group (B), and expansion plus SrR group (C). Each group comprised 12 rats. Neither expansion nor SrR was given to group A. Each rat in groups B and C was set an orthodontic appliance with an initial expansive force of 1 N. The rats in group C were administered with SrR (600 mg.kg(-)(1) body weight) at the same time every day after RME. All the rats were then euthanized in batches on days 4, 7, and 10. The width of the rats' upper jaw was measured. Histological observation was used to section the rats and count the osteoblasts. RESULTS: After the RME, no statistical difference was observed on the rats' upper jaw width in group A (P>0.05). However, the change of upper jaw width in groups B and C presented a statistical significance (P<0.05). By contrast, no statistical difference was observed between groups B and C (P>0.05). The rats' sections were placed under a microscope, and some red fibrous tissues, mesenchymal cells, chondrocytes, and osteoblasts were observed in group A. More red fibrous tissues, mesenchymal cells, and chondrocytes were observed in groups B and C. In addition, more osteoblasts were observed on the edge of mid-palatal suture of the rats. Group C contains more osteoblasts than group B. CONCLUSIONS: RME can expand the mid-palatal suture of rats, which were in the growth development period, and increase the width of dental arch. SrR may promote osteoblast differentiation and hasten new bone formation in the expanded mid-palatal suture. Both conditions accelerate new bone formation and bone deposition calcification, which may be therapeutically beneficial to prevent relapse after RME.
Different doses of strontium ranelate and mechanical vibration modulate distinct responses in the articular cartilage of ovariectomized rats.[Pubmed:28223125]
Osteoarthritis Cartilage. 2017 Jul;25(7):1179-1188.
OBJECTIVE: To investigate the effects of different Strontium Ranelate (SrR) doses alone or in combination with low-intensity and high-frequency mechanical vibration (MV) on articular cartilage in ovariectomized rats. DESIGN: Fifty 6-month-old female Wistar rats underwent ovariectomy (OVX) and after 3 months were divided into: control group (Control); SrR 300 mg/kg/day (SrR300); SrR 625 mg/kg/day (SrR625); MV; SrR 625 mg/kg/day plus MV (SrR625 + MV). The vehicle and the SrR were administered by gavage 7 days/week and vibration (0.6 g/60 Hz) was performed for 20 min/day, 5 days/week. Bone mineral density (BMD) and body composition were evaluated by densitometry. Changes in cartilage were assessed 90 days after treatment by histomorphometry; immunohistochemistry analysis evaluating cell death (caspase-3), tumor necrosis factor-alpha (TNF-alpha), metalloproteinase 9 (MMP-9) and type II collagen; Osteoarthritis Research Society International (OARSI) grading system and glycosaminoglycans (GAGs) analyses. RESULTS: SrR-treated groups exhibited a lower OARSI grade, a smaller number of chondrocyte clusters, increased levels of chondroitin sulfate (CS) and decreased expression of caspase-3. Additionally, compared to all the groups, SrR300 exhibited increased levels of hyaluronic acid (HA). Vibration applied alone or in combination accelerated cartilage degradation, as demonstrated by increased OARSI grade, reduced number of chondrocytes, increased number of clusters, elevated expression of type II collagen and cell death, and was accompanied by decreased amounts of CS and HA; however, MV alone was able to reduce MMP-9. CONCLUSIONS: SrR and vibration modulate distinct responses in cartilage. Combined treatment accelerates degeneration. In contrast, SrR treatment at 300 mg/kg/day attenuates osteoarthritis (OA) progression, improving cartilage matrix quality and preserving cell viability in ovariectomized rats.
Effects of strontium ranelate treatment on osteoblasts cultivated onto scaffolds of trabeculae bovine bone.[Pubmed:28321651]
J Bone Miner Metab. 2018 Jan;36(1):73-86.
Blocks of Bovine bone have shown promising results as implantable scaffolds to promote bone regeneration. Strontium Ranelate (SrR) is both an antiresorptive and an anabolic drug that has been indicated for oral administration to treat osteoporosis. Few studies, however, have investigated the local effects of SrR and its use in association with biomaterials thus far. In this work, we investigated SrR effects in cultures of primary osteoblasts (PO, from Wistar rats calvaria) and immortalized osteoblasts (IO, from MC3T3-E1 cell line) cultivated as a monolayer or in association with scaffolds of bovine bone in mineralized (MBB) and demineralized (DBB) forms. The optimum dose to induce SrR effects on cell viability was established as 0.1 mM. Our results suggested that the local administration of SrR is biocompatible and non-cytotoxic. In addition, SrR appeared to accelerate primary osteoblast cell differentiation by enhancing alkaline phosphatase activity, the expression of osteogenic differentiation markers, the synthesis of the organic matrix, and a decrease of Ca(2+) ions in mineralized nodules. DBB was found to be a better scaffold material to promote PO and IO cell proliferation. Exposing the proteins of the demineralized bone matrix might improve scaffold osteoconductive properties. Our results indicated the importance of further investigation of the administration of SrR at sites of bone repair. The association of SrR and bone grafts suggests the possibility of using SrR as a co-adjuvant for bone tissue bioengineering and in bone regeneration therapies.


