Ro 32-0432 hydrochloridePotent, orally active PKC inhibitor CAS# 151342-35-7 |
- Zoledronic Acid
Catalog No.:BCC1067
CAS No.:118072-93-8
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- Chelerythrine chloride
Catalog No.:BCN8322
CAS No.:3895-92-9
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

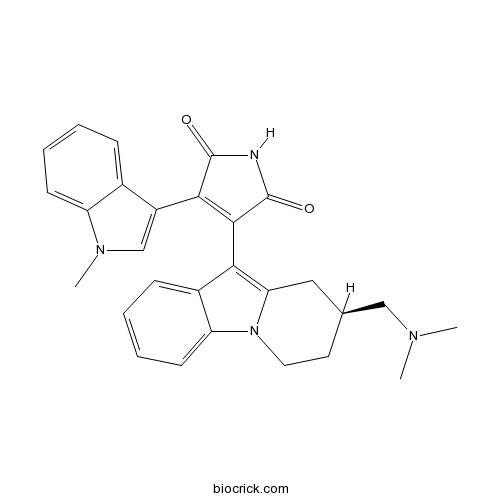
| Cas No. | 151342-35-7 | SDF | Download SDF |
| PubChem ID | 127757 | Appearance | Powder |
| Formula | C28H28N4O2 | M.Wt | 452.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in DMSO with gentle warming | ||
| Chemical Name | 3-[(8S)-8-[(dimethylamino)methyl]-6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl]-4-(1-methylindol-3-yl)pyrrole-2,5-dione | ||
| SMILES | CN1C=C(C2=CC=CC=C21)C3=C(C(=O)NC3=O)C4=C5CC(CCN5C6=CC=CC=C64)CN(C)C | ||
| Standard InChIKey | FXGHOAZJQNLNFD-KRWDZBQOSA-N | ||
| Standard InChI | InChI=1S/C28H28N4O2/c1-30(2)15-17-12-13-32-22-11-7-5-9-19(22)24(23(32)14-17)26-25(27(33)29-28(26)34)20-16-31(3)21-10-6-4-8-18(20)21/h4-11,16-17H,12-15H2,1-3H3,(H,29,33,34)/t17-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective cell-permeable protein kinase C inhibitor. Displays slight selectivity for conventional PKC isoforms over Ca2+ and atypical PKC isoforms; binding affinities for rat isoforms are 9, 28, 31, 37 and 108 nM for PKC's α, βΙ, βΙΙ, γ and ε respectively. Orally available and prevents T cell chronic inflammation in vivo. |

Ro 32-0432 hydrochloride Dilution Calculator

Ro 32-0432 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2099 mL | 11.0497 mL | 22.0994 mL | 44.1989 mL | 55.2486 mL |
| 5 mM | 0.442 mL | 2.2099 mL | 4.4199 mL | 8.8398 mL | 11.0497 mL |
| 10 mM | 0.221 mL | 1.105 mL | 2.2099 mL | 4.4199 mL | 5.5249 mL |
| 50 mM | 0.0442 mL | 0.221 mL | 0.442 mL | 0.884 mL | 1.105 mL |
| 100 mM | 0.0221 mL | 0.1105 mL | 0.221 mL | 0.442 mL | 0.5525 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-O-p-Coumaroyloleanolic acid
Catalog No.:BCN3952
CAS No.:151334-06-4
- Zaleplon
Catalog No.:BCC5197
CAS No.:151319-34-5
- Inokosterone
Catalog No.:BCN3431
CAS No.:15130-85-5
- Genkwanol C
Catalog No.:BCN8012
CAS No.:151283-11-3
- CP 135807
Catalog No.:BCC7774
CAS No.:151272-90-1
- Primin
Catalog No.:BCN2729
CAS No.:15121-94-5
- Poricoic acid AM
Catalog No.:BCN8499
CAS No.:151200-92-9
- Borapetoside F
Catalog No.:BCN6413
CAS No.:151200-50-9
- Borapetoside E
Catalog No.:BCN6571
CAS No.:151200-49-6
- Borapetoside D
Catalog No.:BCN6612
CAS No.:151200-48-5
- 4'-Hydroxy-2,4-dimethoxychalcone
Catalog No.:BCC8708
CAS No.:151135-64-7
- CL 316243 disodium salt
Catalog No.:BCC7091
CAS No.:151126-84-0
- Pseudolarolide B
Catalog No.:BCN8093
CAS No.:151368-43-3
- Estradiol hexahydrobenzoate
Catalog No.:BCC8962
CAS No.:15140-27-9
- Swietemahalactone
Catalog No.:BCN6886
CAS No.:1514669-21-6
- Ampelopsin F
Catalog No.:BCN3305
CAS No.:151487-08-0
- N-[[1-[(2-Nitrophenyl)sulfonyl]-1H-indole-3-yl]methyl]-N-[1-[1-[(2-nitrophenyl)sulfonyl]-1H-indole-3-yl]-2-oxo-2-(tert-butylamino)ethyl]-1-(2-diazo-3-oxobutyryl)-2-oxo-3-methylpiperidine-3beta-carboxamide
Catalog No.:BCC8335
CAS No.:151513-70-1
- Levomefolate calcium
Catalog No.:BCC1702
CAS No.:151533-22-1
- RU 58668
Catalog No.:BCC7608
CAS No.:151555-47-4
- ent-3-Oxokauran-17-oic acid
Catalog No.:BCN1674
CAS No.:151561-88-5
- JJKK 048
Catalog No.:BCC5610
CAS No.:1515855-97-6
- XEN445
Catalog No.:BCC5382
CAS No.:1515856-92-4
- 6-Prenylquercetin-3-methylether
Catalog No.:BCN7992
CAS No.:151649-34-2
- H-D-Ser-OBzl.HCl
Catalog No.:BCC3097
CAS No.:151651-44-4
Ro 32-0432, a selective and orally active inhibitor of protein kinase C prevents T-cell activation.[Pubmed:8114006]
J Pharmacol Exp Ther. 1994 Feb;268(2):922-9.
Several lines of circumstantial evidence support the assumption that protein kinase C (PKC) activation together with elevated levels of cytosolic Ca++ are necessary for T-cell activation and proliferation in response to a physiological stimulus, i.e., MHC class II restricted antigen presentation. By using a potent, cell-permeable and selective inhibitor of PKC, Ro 32-0432, we have tested this hypothesis. Ro 32-0432 inhibits interleukin-2 (IL-2) secretion, IL-2 receptor expression in, and proliferation of, peripheral human T-cells stimulated with phorbol ester together with phytohemagglutin or anti-CD3, but does not inhibit IL-2 induced proliferation in cells already stimulated to express IL-2 receptors. Proliferation of the influenza peptide antigen HA 307-319-specific human T-cell clone (HA27) after exposure to antigen-pulsed autologous presenting cells was also inhibited by Ro 32-0432. Oral administration of Ro 32-0432 inhibited subsequent phorbol ester-induced edema in rats demonstrating the systemic efficacy of the compound to inhibit PKC-driven responses. Induction of more physiologically T-cell driven responses such as host vs. graft responses and the secondary paw swelling in adjuvant-induced arthritis were also inhibited by Ro 32-0432. These data demonstrate the crucial role for PKC in T-cell activation and that selective p.o. bioavailable PKC inhibitors are efficacious in preventing T-cell driven chronic inflammatory responses in vivo. Inhibition of PKC represents an important mechanistic approach to prevent T-cell activation and compounds of this class may have important therapeutic applicability to chronic inflammatory and autoimmune diseases.
Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C.[Pubmed:8373348]
Biochem J. 1993 Sep 1;294 ( Pt 2):335-7.
The protein kinase C (PKC) family of isoenzymes is believed to mediate a wide range of signal-transduction pathways in many different cell types. A series of bisindolylmaleimides have been evaluated as inhibitors of members of the conventional PKC family (PKCs-alpha, -beta, -gamma) and of a representative of the new, Ca(2+)-independent, PKC family, PKC-epsilon. In contrast with the indolocarbazole staurosporine, all the bisindolylmaleimides investigated showed slight selectivity for PKC-alpha over the other isoenzymes examined. In addition, bisindolylmaleimides bearing a conformationally restricted side-chain were less active as inhibitors of PKC-epsilon. Most noticeable of these was Ro 32-0432, which showed a 10-fold selectivity for PKC-alpha and a 4-fold selectivity for PKC-beta I over PKC-epsilon.


