JJKK 048Potent and selective MAGL inhibitor CAS# 1515855-97-6 |
- Cyclopamine
Catalog No.:BCN2964
CAS No.:4449-51-8
- Purmorphamine
Catalog No.:BCC3641
CAS No.:483367-10-8
- GANT61
Catalog No.:BCC1090
CAS No.:500579-04-4
- GANT 58
Catalog No.:BCC1587
CAS No.:64048-12-0
- GDC-0449 (Vismodegib)
Catalog No.:BCC1285
CAS No.:879085-55-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1515855-97-6 | SDF | Download SDF |
| PubChem ID | 76313567 | Appearance | Powder |
| Formula | C23H22N4O5 | M.Wt | 434.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
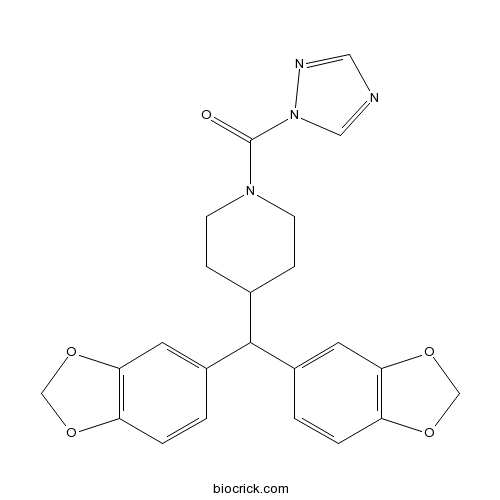
| Chemical Name | [4-[bis(1,3-benzodioxol-5-yl)methyl]piperidin-1-yl]-(1,2,4-triazol-1-yl)methanone | ||
| SMILES | C1CN(CCC1C(C2=CC3=C(C=C2)OCO3)C4=CC5=C(C=C4)OCO5)C(=O)N6C=NC=N6 | ||
| Standard InChIKey | CLSNATLUIXZPMV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H22N4O5/c28-23(27-12-24-11-25-27)26-7-5-15(6-8-26)22(16-1-3-18-20(9-16)31-13-29-18)17-2-4-19-21(10-17)32-14-30-19/h1-4,9-12,15,22H,5-8,13-14H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective monoacylglycerol lipase (MAGL) inhibitor (IC50 = 0.4 nM). Exhibits >13,000 and ~630-fold selectivity for MAGL over FAAH and ABHD6, respectively. Increases brain levels of 2-arachidonoylglycerol (2-AG) levels in mice in vivo. Promotes analgesia in pain models. |

JJKK 048 Dilution Calculator

JJKK 048 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3018 mL | 11.5091 mL | 23.0181 mL | 46.0363 mL | 57.5453 mL |
| 5 mM | 0.4604 mL | 2.3018 mL | 4.6036 mL | 9.2073 mL | 11.5091 mL |
| 10 mM | 0.2302 mL | 1.1509 mL | 2.3018 mL | 4.6036 mL | 5.7545 mL |
| 50 mM | 0.046 mL | 0.2302 mL | 0.4604 mL | 0.9207 mL | 1.1509 mL |
| 100 mM | 0.023 mL | 0.1151 mL | 0.2302 mL | 0.4604 mL | 0.5755 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- ent-3-Oxokauran-17-oic acid
Catalog No.:BCN1674
CAS No.:151561-88-5
- RU 58668
Catalog No.:BCC7608
CAS No.:151555-47-4
- Levomefolate calcium
Catalog No.:BCC1702
CAS No.:151533-22-1
- N-[[1-[(2-Nitrophenyl)sulfonyl]-1H-indole-3-yl]methyl]-N-[1-[1-[(2-nitrophenyl)sulfonyl]-1H-indole-3-yl]-2-oxo-2-(tert-butylamino)ethyl]-1-(2-diazo-3-oxobutyryl)-2-oxo-3-methylpiperidine-3beta-carboxamide
Catalog No.:BCC8335
CAS No.:151513-70-1
- Ampelopsin F
Catalog No.:BCN3305
CAS No.:151487-08-0
- Swietemahalactone
Catalog No.:BCN6886
CAS No.:1514669-21-6
- Estradiol hexahydrobenzoate
Catalog No.:BCC8962
CAS No.:15140-27-9
- Pseudolarolide B
Catalog No.:BCN8093
CAS No.:151368-43-3
- Ro 32-0432 hydrochloride
Catalog No.:BCC7122
CAS No.:151342-35-7
- 3-O-p-Coumaroyloleanolic acid
Catalog No.:BCN3952
CAS No.:151334-06-4
- Zaleplon
Catalog No.:BCC5197
CAS No.:151319-34-5
- Inokosterone
Catalog No.:BCN3431
CAS No.:15130-85-5
- XEN445
Catalog No.:BCC5382
CAS No.:1515856-92-4
- 6-Prenylquercetin-3-methylether
Catalog No.:BCN7992
CAS No.:151649-34-2
- H-D-Ser-OBzl.HCl
Catalog No.:BCC3097
CAS No.:151651-44-4
- SEP-0372814
Catalog No.:BCC6429
CAS No.:1516895-53-6
- 5-Deoxystrigol
Catalog No.:BCN7693
CAS No.:151716-18-6
- 2,2-Dimethyl-8-prenylchromene 6-carboxylic acid
Catalog No.:BCN1675
CAS No.:151731-50-9
- 4,4'-Dihydroxy-2,6-dimethoxydihydrochalcone
Catalog No.:BCN3583
CAS No.:151752-08-8
- Montelukast Sodium
Catalog No.:BCC4680
CAS No.:151767-02-1
- AC 187
Catalog No.:BCC6018
CAS No.:151804-77-2
- cAMPS-Rp, triethylammonium salt
Catalog No.:BCC8082
CAS No.:151837-09-1
- Kinsenoside
Catalog No.:BCN3858
CAS No.:151870-74-5
- 2-Phenylmelatonin
Catalog No.:BCC6748
CAS No.:151889-03-1
In Vivo Characterization of the Ultrapotent Monoacylglycerol Lipase Inhibitor {4-[bis-(benzo[d][1,3]dioxol-5-yl)methyl]-piperidin-1-yl}(1H-1,2,4-triazol-1-yl)m ethanone (JJKK-048).[Pubmed:27451409]
J Pharmacol Exp Ther. 2016 Oct;359(1):62-72.
Monoacylglycerol lipase (MAGL) is a serine hydrolase that acts as a principal degradative enzyme for the endocannabinoid 2-arachidonoylglycerol (2-AG). In addition to terminating the signaling function of 2-AG, MAGL liberates arachidonic acid to be used as a primary source for neuroinflammatory prostaglandin synthesis in the brain. MAGL activity also contributes to cancer pathogenicity by producing precursors for tumor-promoting bioactive lipids. Pharmacological inhibitors of MAGL provide valuable tools for characterization of MAGL and 2-AG signaling pathways. They also hold great therapeutic potential to treat several pathophysiological conditions, such as pain, neurodegenerative disorders, and cancer. We have previously reported piperidine triazole urea, {4-[bis-(benzo[d][1,3]dioxol-5-yl)methyl]-piperidin-1-yl}(1H-1,2,4-triazol-1-yl)m ethanone (JJKK-048), to be an ultrapotent and highly selective inhibitor of MAGL in vitro. Here, we characterize in vivo effects of JJKK-048. Acute in vivo administration of JJKK-048 induced a massive increase in mouse brain 2-AG levels without affecting brain anandamide levels. JJKK-048 appeared to be extremely potent in vivo. Activity-based protein profiling revealed that JJKK-048 maintains good selectivity toward MAGL over other serine hydrolases. Our results are also the first to show that JJKK-048 promoted significant analgesia in a writhing test with a low dose that did not cause cannabimimetic side effects. At a high dose, JJKK-048 induced analgesia both in the writhing test and in the tail-immersion test, as well as hypomotility and hyperthermia, but not catalepsy.
Mutation of Cys242 of human monoacylglycerol lipase disrupts balanced hydrolysis of 1- and 2-monoacylglycerols and selectively impairs inhibitor potency.[Pubmed:24368842]
Mol Pharmacol. 2014 Mar;85(3):510-9.
Considerable progress has been made in recent years in developing selective, potent monoacylglycerol lipase (MAGL) inhibitors. In the investigations of measures to inhibit this enzyme, less attention has been paid to improving our understanding of its catalytic mechanisms or substrate preferences. In our study, we used site-directed mutagenesis, and we show via versatile activity assays combined with molecular modeling that Cys242 and Tyr194, the two opposing amino acid residues in the catalytic cavity of MAGL, play important roles in determining the rate and the isomer preferences of monoacylglycerol hydrolysis. In contrast to wild-type enzymes that hydrolyzed 1- and 2-monoacylglycerols at similar rates, mutation of Cys242 to alanine caused a significant reduction in overall activity (maximal velocity, Vmax), particularly skewing the balanced hydrolysis of isomers to favor the 2-isomer. Molecular modeling studies indicate that this was caused by structural features unfavorable toward 1-isomers as well as impaired recognition of OH-groups in the glycerol moiety. Direct functional involvement of Cys242 in the catalysis was found unlikely due to the remote distance from the catalytic serine. Unlike C242A, mutation of Tyr194 did not bias the hydrolysis of 1- and 2-monoacylglycerols but significantly compromised overall activity. Finally, mutation of Cys242 was also found to impair inhibition of MAGL, especially that by fluorophosphonate derivatives (13- to 63-fold reduction in potency). Taken together, this study provides new experimental and modeling insights into the molecular mechanisms of MAGL-catalyzed hydrolysis of the primary endocannabinoid 2-arachidonoylglycerol and related monoacylglycerols.
Piperazine and piperidine triazole ureas as ultrapotent and highly selective inhibitors of monoacylglycerol lipase.[Pubmed:23521796]
Chem Biol. 2013 Mar 21;20(3):379-90.
Monoacylglycerol lipase (MAGL) terminates the signaling function of the endocannabinoid, 2-arachidonoylglycerol (2-AG). During 2-AG hydrolysis, MAGL liberates arachidonic acid, feeding the principal substrate for the neuroinflammatory prostaglandins. In cancer cells, MAGL redirects lipid stores toward protumorigenic signaling lipids. Thus MAGL inhibitors may have great therapeutic potential. Although potent and increasingly selective MAGL inhibitors have been described, their number is still limited. Here, we have characterized piperazine and piperidine triazole ureas that combine the high potency attributable to the triazole leaving group together with the bulky aromatic benzodioxolyl moiety required for selectivity, culminating in compound JJKK-048 that potently (IC50 < 0.4 nM) inhibited human and rodent MAGL. JJKK-048 displayed low cross-reactivity with other endocannabinoid targets. Activity-based protein profiling of mouse brain and human melanoma cell proteomes suggested high specificity also among the metabolic serine hydrolases.


