Borapetoside ECAS# 151200-49-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 151200-49-6 | SDF | Download SDF |
| PubChem ID | 124578182 | Appearance | Powder |
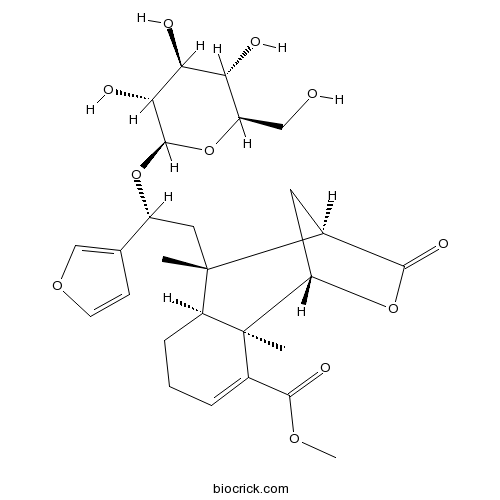
| Formula | C27H36O11 | M.Wt | 536.6 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl (1S,2R,7S,8S,9R)-8-[(2R)-2-(furan-3-yl)-2-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyethyl]-2,8-dimethyl-10-oxo-11-oxatricyclo[7.2.1.02,7]dodec-3-ene-3-carboxylate | ||
| SMILES | CC1(C2CCC=C(C2(C3CC1C(=O)O3)C)C(=O)OC)CC(C4=COC=C4)OC5C(C(C(C(O5)CO)O)O)O | ||
| Standard InChIKey | ZXGKLWUOGQDOTD-IYIXDXQLSA-N | ||
| Standard InChI | InChI=1S/C27H36O11/c1-26(15-9-19(38-24(15)33)27(2)14(23(32)34-3)5-4-6-18(26)27)10-16(13-7-8-35-12-13)36-25-22(31)21(30)20(29)17(11-28)37-25/h5,7-8,12,15-22,25,28-31H,4,6,9-11H2,1-3H3/t15-,16+,17+,18-,19-,20+,21-,22+,25+,26+,27-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1.Borapetoside E has anti-hyperglycemic activity, it can significantly reduce serum glucose levels at dose-dependent manners in alloxan-induced hyperglycemic mice and db/db type 2 diabetic mice. |

Borapetoside E Dilution Calculator

Borapetoside E Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8636 mL | 9.3179 mL | 18.6359 mL | 37.2717 mL | 46.5896 mL |
| 5 mM | 0.3727 mL | 1.8636 mL | 3.7272 mL | 7.4543 mL | 9.3179 mL |
| 10 mM | 0.1864 mL | 0.9318 mL | 1.8636 mL | 3.7272 mL | 4.659 mL |
| 50 mM | 0.0373 mL | 0.1864 mL | 0.3727 mL | 0.7454 mL | 0.9318 mL |
| 100 mM | 0.0186 mL | 0.0932 mL | 0.1864 mL | 0.3727 mL | 0.4659 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Borapetoside D
Catalog No.:BCN6612
CAS No.:151200-48-5
- 4'-Hydroxy-2,4-dimethoxychalcone
Catalog No.:BCC8708
CAS No.:151135-64-7
- CL 316243 disodium salt
Catalog No.:BCC7091
CAS No.:151126-84-0
- 8-(6-Hydroperoxy-3,7-dimethyl-2,7-octadienyloxy)psoralen
Catalog No.:BCN1558
CAS No.:151121-39-0
- 4-Difluoromethoxy-3-hydroxybenzaldehyde
Catalog No.:BCC8706
CAS No.:151103-08-1
- Haloperidol hydrochloride
Catalog No.:BCC4251
CAS No.:1511-16-6
- Moxifloxacin
Catalog No.:BCC4227
CAS No.:151096-09-2
- L-701,252
Catalog No.:BCC6755
CAS No.:151057-13-5
- DOXO-EMCH
Catalog No.:BCC1537
CAS No.:151038-96-9
- H-Phe-OtBu.HCl
Catalog No.:BCC3011
CAS No.:15100-75-1
- Boc-Lys(Boc)-OH.DCHA
Catalog No.:BCC3413
CAS No.:15098-69-8
- 7,8-Didehydrocimigenol
Catalog No.:BCN3343
CAS No.:150972-72-8
- Borapetoside F
Catalog No.:BCN6413
CAS No.:151200-50-9
- Poricoic acid AM
Catalog No.:BCN8499
CAS No.:151200-92-9
- Primin
Catalog No.:BCN2729
CAS No.:15121-94-5
- CP 135807
Catalog No.:BCC7774
CAS No.:151272-90-1
- Genkwanol C
Catalog No.:BCN8012
CAS No.:151283-11-3
- Inokosterone
Catalog No.:BCN3431
CAS No.:15130-85-5
- Zaleplon
Catalog No.:BCC5197
CAS No.:151319-34-5
- 3-O-p-Coumaroyloleanolic acid
Catalog No.:BCN3952
CAS No.:151334-06-4
- Ro 32-0432 hydrochloride
Catalog No.:BCC7122
CAS No.:151342-35-7
- Pseudolarolide B
Catalog No.:BCN8093
CAS No.:151368-43-3
- Estradiol hexahydrobenzoate
Catalog No.:BCC8962
CAS No.:15140-27-9
- Swietemahalactone
Catalog No.:BCN6886
CAS No.:1514669-21-6
Clerodane Diterpenoids with Anti-hyperglycemic Activity from Tinospora crispa.[Pubmed:27752986]
Nat Prod Bioprospect. 2016 Oct;6(5):247-255.
Four new clerodane diterpenoids, tinosporols A-C (2-4) and tinosporoside A (5), together with six known analogues were isolated from the vines of Tinospora crispa. Their structures were established by extensive spectroscopic analysis. The relative configuration at C-12 in the known diterpenoid Borapetoside E (1), the major component of the plant, was firstly established with the aid of molecular model. Compound 1 significantly reduced serum glucose levels at dose-dependent manners in alloxan-induced hyperglycemic mice and db/db type 2 diabetic mice.
Crude extract and purified components isolated from the stems of Tinospora crispa exhibit positive inotropic effects on the isolated left atrium of rats.[Pubmed:23778316]
J Ethnopharmacol. 2013 Aug 26;149(1):123-32.
ETHNOPHARMACOLOGICAL RELEVANCE: Tinospora crispa has been used in folkloric medicine for the control of blood pressure. We previously found that an extract of Tinospora crispa and its constituents effect the heart rate and blood pressure in anesthetized rats. AIM OF THE STUDY: The aim was to investigate the effects and mechanisms of the Tinospora crispa extract and bioactive components on the rat isolated left atria. MATERIALS AND METHODS: Air-dried stems of Tinospora crispa were extracted with water, followed by partitioning with chloroform, ethyl acetate, and finally by n-butanol. The n-butanol soluble material was concentrated and dried under reduced pressure and lyophilized to obtain a crude powder (Tinospora crispa extract). The active components of Tinospora crispa extract were separated by column chromatography and preparative HPLC. The effects and mechanisms of the n-butanol extract and the bioactive purified components (adenine, uridine, adenosine, salsolinol, tyramine, higenamine, syringin, (-)-litcubinine, borapetoside A, borapetoside B, borapetoside D and Borapetoside E) were studied in isolated left atria from normal and reserpinized rats. RESULTS: Tinospora crispa extract caused an increase in the force of contraction of the electrical field stimulated left atrium. This effect was inhibited by propranolol, atenolol, ICI-118,551, phentolamine and atropine. The positive inotropic effect on the reserpenized isolated left atrium of the Tinospora crispa extract was significantly inhibited by propranolol, atenolol and ICI-118,551. Phentolamine, on the other hand, caused potentiation and the effect was inhibited when propranolol was also added. Higenamine caused an increase in the force of contraction of the electrical field stimulated left atrium and this effect was significantly inhibited by ICI-118,551 and atenolol but not by phentolamine. Reserpine did not significantly shift the concentration-response curve (C-R curve) of the inotropic effect of the higenamine. ICI-118,551 and atenolol caused a parallel shift of the C-R curve to the right of about 8 and 33 fold, respectively. At low concentrations salsolinol caused a slight increase in the force of contraction of the left atrium, but at higher concentrations a decrease was observed. The negative inotropic effect of salsolinol was significantly inhibited by propranolol and atropine. In the reserpinized isolated left atrium, the negative inotropic effect of salsolinol was potentiated and again this effect was significantly inhibited by propranolol and atropine. Tyramine caused a positive inotropic effect, and this effect was inhibited by propranolol or by pretreatment of the rat with reserpine. Adenosine caused a negative inotropic effect, while uridine caused a slight positive inotropic effect on the left atrium. This effect was significantly inhibited by DPCPX. CONCLUSIONS: Crude extract of Tinospora crispa exert a positive inotropic effect on the electrical field stimulated isolated left atria that results from the concerted action of 5 bioactive compounds: higenamine, salsolinol, tyramine, adenosine and uridine. Higenamine, salsolinol (at low concentration) and tyramine acted via the adrenergic receptors to increase the force of the atrial contraction, whereas a high concentration of salsolinol acted indirectly by stimulating the release of acetylcholine. Adenosine and uridine acted via the purinergic pathways to cause negative inotropic effects on the isolated left atria.


