L-701,252CAS# 151057-13-5 |
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- Semagacestat (LY450139)
Catalog No.:BCC3610
CAS No.:425386-60-3
- Flurizan
Catalog No.:BCC2342
CAS No.:51543-40-9
- E 2012
Catalog No.:BCC1540
CAS No.:870843-42-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 151057-13-5 | SDF | Download SDF |
| PubChem ID | 54687453 | Appearance | Powder |
| Formula | C13H10ClNO3 | M.Wt | 263.68 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO | ||
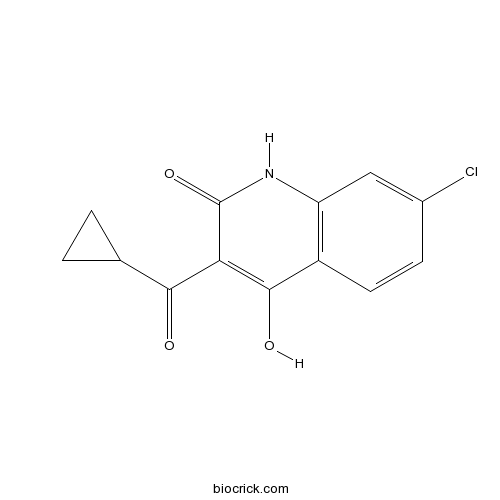
| Chemical Name | 7-chloro-3-(cyclopropanecarbonyl)-4-hydroxy-1H-quinolin-2-one | ||
| SMILES | C1CC1C(=O)C2=C(C3=C(C=C(C=C3)Cl)NC2=O)O | ||
| Standard InChIKey | MXEFWCFPCLDOOG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H10ClNO3/c14-7-3-4-8-9(5-7)15-13(18)10(12(8)17)11(16)6-1-2-6/h3-6H,1-2H2,(H2,15,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | An antagonist at the glycine-NMDA site (IC50 = 420 nM). Also a potent systemic anticonvulsant. |

L-701,252 Dilution Calculator

L-701,252 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7925 mL | 18.9624 mL | 37.9248 mL | 75.8495 mL | 94.8119 mL |
| 5 mM | 0.7585 mL | 3.7925 mL | 7.585 mL | 15.1699 mL | 18.9624 mL |
| 10 mM | 0.3792 mL | 1.8962 mL | 3.7925 mL | 7.585 mL | 9.4812 mL |
| 50 mM | 0.0758 mL | 0.3792 mL | 0.7585 mL | 1.517 mL | 1.8962 mL |
| 100 mM | 0.0379 mL | 0.1896 mL | 0.3792 mL | 0.7585 mL | 0.9481 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- DOXO-EMCH
Catalog No.:BCC1537
CAS No.:151038-96-9
- H-Phe-OtBu.HCl
Catalog No.:BCC3011
CAS No.:15100-75-1
- Boc-Lys(Boc)-OH.DCHA
Catalog No.:BCC3413
CAS No.:15098-69-8
- 7,8-Didehydrocimigenol
Catalog No.:BCN3343
CAS No.:150972-72-8
- 2,4,4'-Trihydroxydihydrochalcone
Catalog No.:BCN7365
CAS No.:15097-74-2
- Tirofiban hydrochloride monohydrate
Catalog No.:BCC2003
CAS No.:150915-40-5
- D-allo-Ile-OH
Catalog No.:BCC2966
CAS No.:1509-35-9
- Aurantiamide benzoate
Catalog No.:BCN8043
CAS No.:150881-02-0
- Euonymine
Catalog No.:BCN3084
CAS No.:150881-01-9
- Bis(phenylacetyl) disulfide
Catalog No.:BCC8887
CAS No.:15088-78-5
- Uralenol-3-methylether
Catalog No.:BCN7993
CAS No.:150853-98-8
- Micromelin
Catalog No.:BCN1672
CAS No.:15085-71-9
- Moxifloxacin
Catalog No.:BCC4227
CAS No.:151096-09-2
- Haloperidol hydrochloride
Catalog No.:BCC4251
CAS No.:1511-16-6
- 4-Difluoromethoxy-3-hydroxybenzaldehyde
Catalog No.:BCC8706
CAS No.:151103-08-1
- 8-(6-Hydroperoxy-3,7-dimethyl-2,7-octadienyloxy)psoralen
Catalog No.:BCN1558
CAS No.:151121-39-0
- CL 316243 disodium salt
Catalog No.:BCC7091
CAS No.:151126-84-0
- 4'-Hydroxy-2,4-dimethoxychalcone
Catalog No.:BCC8708
CAS No.:151135-64-7
- Borapetoside D
Catalog No.:BCN6612
CAS No.:151200-48-5
- Borapetoside E
Catalog No.:BCN6571
CAS No.:151200-49-6
- Borapetoside F
Catalog No.:BCN6413
CAS No.:151200-50-9
- Poricoic acid AM
Catalog No.:BCN8499
CAS No.:151200-92-9
- Primin
Catalog No.:BCN2729
CAS No.:15121-94-5
- CP 135807
Catalog No.:BCC7774
CAS No.:151272-90-1
Evaluation of glycine site antagonists of the NMDA receptor in global cerebral ischaemia.[Pubmed:10082862]
Brain Res. 1999 Feb 20;819(1-2):65-74.
In the present studies we have investigated the effects of a range of glycine site antagonists of the N-methyl-d-aspartate (NMDA) receptor in the gerbil model of global cerebral ischaemia. The compounds tested were (+)-3-amino-1-hydroxy-2-pyrrolidone (HA 966, 15 mg/kg), 7-chloro-4-hydroxy-3-(3-phenoxy)phenyl-2(H)-quinolinone) (L-701,324, 40 mg/kg), 7-chloro-3-(cyclopropylcarbonyl)-4-hydroxy-2(1H)-quinolinone) (L-701, 252, 50 mg/kg), (3-(3-hydroxyphenyl)prop-2-ynyl 7-chloro-4 hydroxy-2(1H)-quinolone-3-carboxylate) (L-701,273, 50 mg/kg), 5-nitro-6,7-dichloro-2,3-quinoxalinedione (ACEA 1021, 25 mg/kg) and [(E)-3[(phenylcarbamoyl) ethenyl]-4,6-dichloroindole-2-carboxylic acid sodium salt (GV 150526A, 40 mg/kg). All compounds were administered via the i.p. route 30 min before and again at 2 h 30 min after 5 min bilateral carotid artery occlusion (BCAO) in the gerbil. For comparison we also evaluated a non-competitive NMDA antagonist, (5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a, d]cyclohepten-5,10-imine (MK-801, 2 mg/kg) and an alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) antagonist, (3S,4aR, 6R, 8aR)-6-[2-(1(2)H-tetrazole-5-yl)]decahydroisoquinoline-3-car boxylic acid (LY293558, 20 mg/kg). In the present studies L-701,252, L-701, 324 and L-701,273 provided a small degree of neuroprotection. ACEA 1021, GV 150526A and HA 966 failed to provide any neuroprotection, while MK-801 provided significant (20%) protection. In contrast LY293558 provided good (55%) neuroprotection. These results indicate that glycine site antagonists and competitive NMDA antagonists provide a small degree of neuroprotection in global cerebral ischaemia. In contrast, AMPA receptor antagonists provide more robust neuroprotection in global cerebral ischaemia.
3-Acyl-4-hydroxyquinolin-2(1H)-ones. Systemically active anticonvulsants acting by antagonism at the glycine site of the N-methyl-D-aspartate receptor complex.[Pubmed:8230129]
J Med Chem. 1993 Oct 29;36(22):3386-96.
Most full antagonists at the glycine site of the NMDA receptor contain a carboxylic acid, which we believe to be detrimental to penetration of the blood-brain barrier. By consideration of a pharmacophore, novel antagonists at this site have been designed in which the anionic functionality is a vinylogous acid, in the form of a 4-hydroxyquinolin-2(1H)-one. In this series, a 3-substituent is necessary for binding, and correct manipulation of this group leads to compounds such as the 3-(3-hydroxyphenyl)propargyl ester 24 (L-701,273), with an IC50 for displacement of [3H]-L-689,560 binding of 0.17 microM and Kb against NMDA in the cortical slice of 1.39 microM. Compounds were tested for their ability to prevent audiogenic seizure in DBA/2 mice; the most potent compound in this series is the cyclopropyl ketone 42 (L-701,252), with an ED50 of 4.1 mg/kg ip. A model is proposed for binding to the glycine site, in which an important interaction is of a putative receptor cation with the pi-system of the 3-substituent.


