Tirofiban hydrochloride monohydrateGlycoprotein IIb/IIIa inhibitor CAS# 150915-40-5 |
- Tirasemtiv
Catalog No.:BCC5183
CAS No.:1005491-05-3
- Methylcobalamin
Catalog No.:BCC5188
CAS No.:13422-55-4
- TPT-260 Dihydrochloride
Catalog No.:BCC5172
CAS No.:2076-91-7
- Miglustat hydrochloride
Catalog No.:BCC5186
CAS No.:210110-90-0
- Amifampridine
Catalog No.:BCC5185
CAS No.:54-96-6
- Miglustat
Catalog No.:BCC5187
CAS No.:72599-27-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

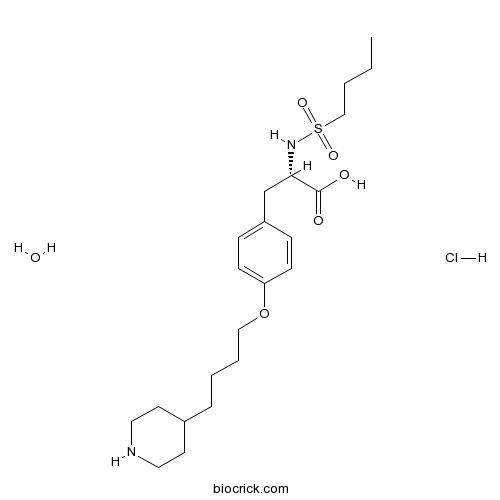
| Cas No. | 150915-40-5 | SDF | Download SDF |
| PubChem ID | 60946 | Appearance | Powder |
| Formula | C22H39ClN2O6S | M.Wt | 495.07 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 12 mg/mL (24.24 mM; Need ultrasonic and warming) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | (2S)-2-(butylsulfonylamino)-3-[4-(4-piperidin-4-ylbutoxy)phenyl]propanoic acid;hydrate;hydrochloride | ||
| SMILES | CCCCS(=O)(=O)NC(CC1=CC=C(C=C1)OCCCCC2CCNCC2)C(=O)O.O.Cl | ||
| Standard InChIKey | HWAAPJPFZPHHBC-FGJQBABTSA-N | ||
| Standard InChI | InChI=1S/C22H36N2O5S.ClH.H2O/c1-2-3-16-30(27,28)24-21(22(25)26)17-19-7-9-20(10-8-19)29-15-5-4-6-18-11-13-23-14-12-18;;/h7-10,18,21,23-24H,2-6,11-17H2,1H3,(H,25,26);1H;1H2/t21-;;/m0../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tirofiban hydrochloride monohydrate is a potent non-peptide, glycoprotein IIb/IIIa (integrins alphaIIbbetaIII) antagonist
IC50 value:
Target: integrin IIb/IIIa
Tirofiban hydrochloride monohydrate blocks platelet aggregation and thrombus formation. Tirofiban is an antithrombotic used in the treatment of unstable angina.
Tirofiban, in a concentration-dependent manner reduced platelet aggregation evoked by ADP (IC50 approximately 70 ng/ml), collagen (IC50 approximately 200 ng/ml), and thrombin (IC50 approximately 5,000 ng/ml). References: | |||||

Tirofiban hydrochloride monohydrate Dilution Calculator

Tirofiban hydrochloride monohydrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0199 mL | 10.0996 mL | 20.1992 mL | 40.3983 mL | 50.4979 mL |
| 5 mM | 0.404 mL | 2.0199 mL | 4.0398 mL | 8.0797 mL | 10.0996 mL |
| 10 mM | 0.202 mL | 1.01 mL | 2.0199 mL | 4.0398 mL | 5.0498 mL |
| 50 mM | 0.0404 mL | 0.202 mL | 0.404 mL | 0.808 mL | 1.01 mL |
| 100 mM | 0.0202 mL | 0.101 mL | 0.202 mL | 0.404 mL | 0.505 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tirofiban hydrochloride monohydrate is a potent non-peptide, glycoprotein IIb/IIIa inhibitor. Tirofiban hydrochloride monohydrate blocks platelet aggregation and thrombus formation. Tirofiban is an antithrombotic used in the treatment of unstable angina.
- D-allo-Ile-OH
Catalog No.:BCC2966
CAS No.:1509-35-9
- Aurantiamide benzoate
Catalog No.:BCN8043
CAS No.:150881-02-0
- Euonymine
Catalog No.:BCN3084
CAS No.:150881-01-9
- Bis(phenylacetyl) disulfide
Catalog No.:BCC8887
CAS No.:15088-78-5
- Uralenol-3-methylether
Catalog No.:BCN7993
CAS No.:150853-98-8
- Micromelin
Catalog No.:BCN1672
CAS No.:15085-71-9
- Boc-Orn(Fmoc)-OH
Catalog No.:BCC3429
CAS No.:150828-96-9
- EGF816
Catalog No.:BCC6428
CAS No.:1508250-71-2
- Nitenpyram
Catalog No.:BCC5559
CAS No.:150824-47-8
- 2,3,24-Trihydroxyolean-12-en-28-oic acid
Catalog No.:BCN1559
CAS No.:150821-16-2
- Retigabine dihydrochloride
Catalog No.:BCC1890
CAS No.:150812-13-8
- Retigabine
Catalog No.:BCC6427
CAS No.:150812-12-7
- 2,4,4'-Trihydroxydihydrochalcone
Catalog No.:BCN7365
CAS No.:15097-74-2
- 7,8-Didehydrocimigenol
Catalog No.:BCN3343
CAS No.:150972-72-8
- Boc-Lys(Boc)-OH.DCHA
Catalog No.:BCC3413
CAS No.:15098-69-8
- H-Phe-OtBu.HCl
Catalog No.:BCC3011
CAS No.:15100-75-1
- DOXO-EMCH
Catalog No.:BCC1537
CAS No.:151038-96-9
- L-701,252
Catalog No.:BCC6755
CAS No.:151057-13-5
- Moxifloxacin
Catalog No.:BCC4227
CAS No.:151096-09-2
- Haloperidol hydrochloride
Catalog No.:BCC4251
CAS No.:1511-16-6
- 4-Difluoromethoxy-3-hydroxybenzaldehyde
Catalog No.:BCC8706
CAS No.:151103-08-1
- 8-(6-Hydroperoxy-3,7-dimethyl-2,7-octadienyloxy)psoralen
Catalog No.:BCN1558
CAS No.:151121-39-0
- CL 316243 disodium salt
Catalog No.:BCC7091
CAS No.:151126-84-0
- 4'-Hydroxy-2,4-dimethoxychalcone
Catalog No.:BCC8708
CAS No.:151135-64-7
Effects of Tirofiban on Random Skin Flap Survival in Rats.[Pubmed:28992645]
J Reconstr Microsurg. 2018 Feb;34(2):138-144.
BACKGROUND: Tirofiban is a glycoprotein IIb/IIIa receptor antagonist that is widely used clinically. In the present study, we investigated whether tirofiban promotes flap survival in rat random skin flap model. METHODS: "McFarlane flaps" models were developed in 60 male rats. The rats were divided into a tirofiban-treated group (n = 30) and a saline-treated group (n = 30). The flap surviving rate was calculated 7 days after surgery. Tissue samples were collected and subjected to histopathological evaluation. Lead oxide-gelatin angiography and immunohistochemical staining analysis were taken to evaluate angiogenesis. Analysis of oxidative stress was performed by measuring the activity of superoxide dismutase (SOD) and malondialdehyde (MDA). RESULTS: Compared with controls, the tirofiban-treated groups exhibited significantly larger mean areas of flap survival, significantly increased SOD activity, and vascular endothelial growth factor (VEGF) expression, and significantly reduced MDA level. Hematoxylin and eosin staining revealed that naringin promoted angiogenesis and inhibited inflammation. CONCLUSION: These findings demonstrate that tirofiban increases flap survival of random skin flaps in rats.
Tirofiban facilitates the reperfusion process during endovascular thrombectomy in ICAS.[Pubmed:28912883]
Exp Ther Med. 2017 Oct;14(4):3314-3318.
The aim of the present study was to assess the use of tirofiban injections for rescue therapy following artery reocclusion due to intra-luminal thrombosis during endovascular thrombectomy in patients with acute ischemic stroke (AIS). A total of seven cases of patients treated with adjunctive tirofiban injections following failed endovascular thrombectomy due to instant intra-luminal thrombosis were retrospectively assessed. A Solitaire stent was used as the primary thrombectomy device in all patients. Tirofiban was injected intra-arterially via a temporarily deployed Solitaire stent with continuous intravenous infusion for the subsequent 24 h; half of the conventionally recommended dose was employed. Outcome measures included angiographic reperfusion (mTICI), symptomatic intracranial hemorrhage, mortality and functional independence at 90 days (modified Rankin Scale, 0-2). Six patients had occlusions in the middle cerebral artery and one patient had occlusions in the basilar artery. Of the seven patients, five exhibited successful reperfusion (mTICI 2b-3) and achieved functional independence following 90 days. Reperfusion failed in the remaining two patients, who succumbed within 90 days of therapy. No intracranial or extracranial hemorrhage cases were identified. The results of the present study suggest that tirofiban facilitates reperfusion and ameliorates long-term prognosis in patients with AIS undergoing endovascular thrombectomy, and may be safe for those receiving intravenous tissue plasminogen activator therapy.
Endovascular Treatment of Ruptured Vertebrobasilar Dissecting Aneurysms Using Flow Diversion Embolization Devices: Single-Institution Experience.[Pubmed:28987840]
World Neurosurg. 2018 Jan;109:e164-e169.
OBJECTIVE: Treatment of ruptured posterior circulation dissecting aneurysms is technically challenging with potentially high morbidity and mortality. We sought to assess the safety and feasibility of using a flow-diversion device (FDD) and a specific acute antiplatelet aggregation protocol in the management of ruptured dissecting aneurysms. METHODS: Subjects with ruptured dissecting aneurysms treated during a 3-year period were retrospectively identified from a prospective registry. Intraoperative complications, morbidity, and mortality were recorded. Tirofiban maintenance infusion without bolus was administered intravenously immediately after deployment of the FDD, and almost all patients were loaded with dual antiplatelet (aspirin and clopidogrel) post procedure. Clinical follow-up evaluation and modified Rankin Scale were assessed. RESULTS: Nine subjects with ruptured posterior circulation dissecting aneurysms were treated with an FDD: 5 vertebral artery, 2 basilar artery, and 2 posterior inferior cerebellar artery aneurysms. Average World Federation of Neurosurgical Societies score was 2 (range 1-5). Seven patients had external ventricular drain placed acutely for hydrocephalus. Eight patients received tirofiban infusion without bolus after FDD. No intraoperative complications occurred. Two subjects developed asymptomatic intraparenchymal hemorrhage found on surveillance noncontrast computed tomography. One subject suffered a major intraparenchymal hemorrhage and died a few days post intervention after additional anticoagulation was started for a left ventricular assist device. Follow-up modified Rankin Scale within 12 months was 0 in 3 subjects, 1 in 3 subjects, 2 in 1 subject, and 4 in 1. CONCLUSIONS: Treatment of dissecting posterior circulation aneurysms with FDDs is feasible and a potential alternative to deconstructive techniques.
Force-activatable biosensor enables single platelet force mapping directly by fluorescence imaging.[Pubmed:28915383]
Biosens Bioelectron. 2018 Feb 15;100:192-200.
Integrin-transmitted cellular forces are critical for platelet adhesion, activation, aggregation and contraction during hemostasis and thrombosis. Measuring and mapping single platelet forces are desired in both research and clinical applications. Conventional force-to-strain based cell traction force microscopies have low resolution which is not ideal for cellular force mapping in small platelets. To enable platelet force mapping with submicron resolution, we developed a force-activatable biosensor named integrative tension sensor (ITS) which directly converts molecular tensions to fluorescent signals, therefore enabling cellular force mapping directly by fluorescence imaging. With ITS, we mapped cellular forces in single platelets at 0.4microm resolution. We found that platelet force distribution has strong polarization which is sensitive to treatment with the anti-platelet drug tirofiban, suggesting that the ITS force map can report anti-platelet drug efficacy. The ITS also calibrated integrin molecular tensions in platelets and revealed two distinct tension levels: 12-54 piconewton (nominal values) tensions generated during platelet adhesion and tensions above 54 piconewton generated during platelet contraction. Overall, the ITS is a powerful biosensor for the study of platelet mechanobiology, and holds great potential in antithrombotic drug development and assessing platelet activity in health and disease.
Intralesional versus intracoronary administration of glycoprotein IIb/IIIa inhibitors during percutaneous coronary intervention in patients with acute coronary syndromes: A meta-analysis of randomized controlled trials.[Pubmed:28984776]
Medicine (Baltimore). 2017 Oct;96(40):e8223.
BACKGROUND: Glycoprotein IIb/IIIa inhibitors (GPIs) have been regarded as an adjuvant regimen to deal with no-reflow. However, whether intralesional (IL) administration of GPIs improves myocardial reperfusion without increasing bleeding in patients with acute coronary syndrome (ACS) compared with intracoronary (IC) administration has not been well addressed. Our meta-analysis aimed to evaluate the efficacy and safety of IL versus IC administration of GPIs for patients with ACS during percutaneous coronary intervention. METHODS: We systematically searched Medline, Embase, the Cochrane Central Register of Controlled Trials, and Cambridge Scientific Abstracts from January 2007 to May 2017. Thrombolysis in Myocardial Infarction (TIMI) 3 flow, corrected TIMI frame count (CTFC), and complete ST-segment resolution (>70%) were selected as the primary outcomes. Major adverse cardiac events (MACEs) were the secondary outcome, and major bleeding complications were the safety outcome. Data analysis was conducted using the Review Manager 5.3 software. RESULTS: Six randomized controlled trials were included in our meta-analysis. Compared with IC, IL obtained better results in terms of TIMI grade 3 flow [odds ratio (OR) 2.29; 95% confidence intervals (CIs) 1.31-4.01; P = .004], CTFC [weighted mean difference (WMD) -4.63; 95% CI -8.82 to -0.43; P = .03], and complete ST-segment resolution (OR 1.55; 95% CI 1.12-2.14; P = .008). There was a trend toward decreased MACE in the IL administration groups, which was not of statistical significance (OR 0.63; 95% CI 0.30-1.31; P = .22). No significant difference was found between the two groups in terms of in-hospital major bleeding events (OR 2.52; 95% CI .66 to 9.62; P = .18). CONCLUSION: IL administration yielded favorable outcomes in terms of myocardial tissue reperfusion as evidenced by the improved TIMI flow grade, CTFC, complete ST-segment resolution, and decreased MACE without increasing in-hospital major bleeding events. The IL administration of GPIs can be recommended as the preferred regimen to guard against no-reflow.


