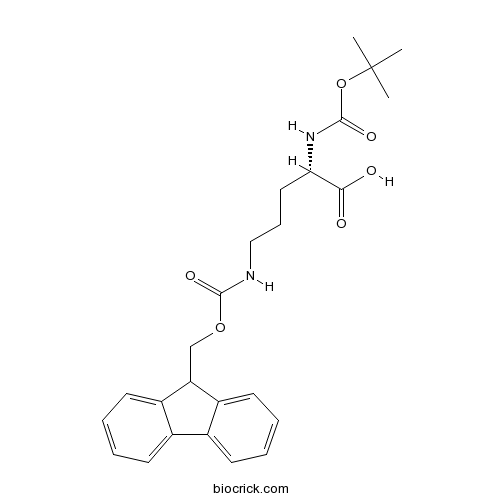
Boc-Orn(Fmoc)-OHCAS# 150828-96-9 |
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Z-WEHD-FMK
Catalog No.:BCC1139
CAS No.:210345-00-9
- Z-LEHD-FMK
Catalog No.:BCC5117
CAS No.:210345-04-3
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- Apoptosis Activator 2
Catalog No.:BCC2099
CAS No.:79183-19-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 150828-96-9 | SDF | Download SDF |
| PubChem ID | 16211325 | Appearance | Powder |
| Formula | C25H30N2O6 | M.Wt | 454.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-5-(9H-fluoren-9-ylmethoxycarbonylamino)-2-[(2-methylpropan-2-yl)oxycarbonylamino]pentanoic acid | ||
| SMILES | CC(C)(C)OC(=O)NC(CCCNC(=O)OCC1C2=CC=CC=C2C3=CC=CC=C13)C(=O)O | ||
| Standard InChIKey | YEBWACZYMHWWEK-NRFANRHFSA-N | ||
| Standard InChI | InChI=1S/C25H30N2O6/c1-25(2,3)33-24(31)27-21(22(28)29)13-8-14-26-23(30)32-15-20-18-11-6-4-9-16(18)17-10-5-7-12-19(17)20/h4-7,9-12,20-21H,8,13-15H2,1-3H3,(H,26,30)(H,27,31)(H,28,29)/t21-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Boc-Orn(Fmoc)-OH Dilution Calculator

Boc-Orn(Fmoc)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2002 mL | 11.0011 mL | 22.0022 mL | 44.0044 mL | 55.0055 mL |
| 5 mM | 0.44 mL | 2.2002 mL | 4.4004 mL | 8.8009 mL | 11.0011 mL |
| 10 mM | 0.22 mL | 1.1001 mL | 2.2002 mL | 4.4004 mL | 5.5006 mL |
| 50 mM | 0.044 mL | 0.22 mL | 0.44 mL | 0.8801 mL | 1.1001 mL |
| 100 mM | 0.022 mL | 0.11 mL | 0.22 mL | 0.44 mL | 0.5501 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Boc-Orn(Fmoc)-OH
- EGF816
Catalog No.:BCC6428
CAS No.:1508250-71-2
- Nitenpyram
Catalog No.:BCC5559
CAS No.:150824-47-8
- 2,3,24-Trihydroxyolean-12-en-28-oic acid
Catalog No.:BCN1559
CAS No.:150821-16-2
- Retigabine dihydrochloride
Catalog No.:BCC1890
CAS No.:150812-13-8
- Retigabine
Catalog No.:BCC6427
CAS No.:150812-12-7
- Tropicamide
Catalog No.:BCC4574
CAS No.:1508-75-4
- Oxybutynin chloride
Catalog No.:BCC4149
CAS No.:1508-65-2
- Gap19
Catalog No.:BCC5599
CAS No.:1507930-57-5
- Drynachromoside A
Catalog No.:BCN7891
CAS No.:1507388-29-5
- Calyxamine B
Catalog No.:BCN1671
CAS No.:150710-72-8
- 5-Methoxycanthin-6-one
Catalog No.:BCN3326
CAS No.:15071-56-4
- Tolvaptan
Catalog No.:BCC5096
CAS No.:150683-30-0
- Micromelin
Catalog No.:BCN1672
CAS No.:15085-71-9
- Uralenol-3-methylether
Catalog No.:BCN7993
CAS No.:150853-98-8
- Bis(phenylacetyl) disulfide
Catalog No.:BCC8887
CAS No.:15088-78-5
- Euonymine
Catalog No.:BCN3084
CAS No.:150881-01-9
- Aurantiamide benzoate
Catalog No.:BCN8043
CAS No.:150881-02-0
- D-allo-Ile-OH
Catalog No.:BCC2966
CAS No.:1509-35-9
- Tirofiban hydrochloride monohydrate
Catalog No.:BCC2003
CAS No.:150915-40-5
- 2,4,4'-Trihydroxydihydrochalcone
Catalog No.:BCN7365
CAS No.:15097-74-2
- 7,8-Didehydrocimigenol
Catalog No.:BCN3343
CAS No.:150972-72-8
- Boc-Lys(Boc)-OH.DCHA
Catalog No.:BCC3413
CAS No.:15098-69-8
- H-Phe-OtBu.HCl
Catalog No.:BCC3011
CAS No.:15100-75-1
- DOXO-EMCH
Catalog No.:BCC1537
CAS No.:151038-96-9
An unnatural amino acid that induces beta-sheet folding and interaction in peptides.[Pubmed:11982357]
J Am Chem Soc. 2002 May 8;124(18):4972-3.
This paper introduces a unique amino acid that can readily be incorporated into peptides to make them fold into beta-sheetlike structures that dimerize through beta-sheet interactions. This new amino acid, Orn(i-PrCO-Hao), consists of an ornithine residue with the beta-strand-mimicking amino acid Hao [J. Am. Chem. Soc. 2000, 122, 7654-7661] attached to its side chain. When Orn(i-PrCO-Hao) is incorporated into a peptide, or appended to its N-terminus, the Hao group hydrogen bonds to the three subsequent residues to form a beta-sheetlike structure. The amino acid Orn(i-PrCO-Hao) is readily used in peptide synthesis as its Fmoc derivative, Fmoc-Orn(i-PrCO-Hao)-OH (3). Fmoc-Orn(i-PrCO-Hao)-OH behaves like a regular amino acid in peptide synthesis and was uneventfully incorporated into the peptide o-anisoyl-Val-Orn(i-PrCO-Hao)-Phe-Ile-Leu-NHMe (4) through standard automated Fmoc solid-phase peptide synthesis, with DIC and HOAt as the coupling agent for Fmoc-Orn(i-PrCO-Hao)-OH and o-anisic acid and HATU as the coupling agent for all other couplings. A second synthetic strategy was developed to facilitate the preparation of peptides with N-terminal Orn(i-PrCO-Hao) residues, which avoids the need for the preparation of Fmoc-Orn(i-PrCO-Hao)-OH. In this strategy, Boc-Orn(Fmoc)-OH is used as the penultimate amino acid in the peptide synthesis, and i-PrCO-Hao-OH (2) is used as the final amino acid. N-Terminal Orn(i-PrCO-Hao) peptide H-Orn(i-PrCO-Hao)-Phe-Ile-Leu-NHMe.TFA (5) was prepared in a fashion similar to that for 4, using DIC and HOAt as the coupling agent for i-PrCO-Hao-OH and HATU as the coupling agent for all other couplings. 1H NMR transverse-ROESY, coupling constant, and chemical shift studies establish that peptide 4 forms a dimeric beta-sheetlike structure in CDCl3 solution. The 1H NMR studies also suggest that the ornithine unit adopts a well-defined turn conformation. Analogous 1H NMR studies of peptide 5 indicate that this TFA salt folds but does not dimerize in CD3OD solution. Collectively, these synthetic and spectroscopic studies establish that the amino acid Orn(i-PrCO-Hao) induces beta-sheet structure and interactions in peptides in suitable organic solvents. Unlike the Hao amino acid, which acts as a prosthetic to replace three residues of the peptide strand, the Orn(i-PrCO-Hao) amino acid acts as a splint that helps enforce a beta-sheetlike structure without replacing the residues and their side chains. This feature of Orn(i-PrCO-Hao) is important, because it allows the creation of beta-sheet structure with minimal perturbation of the peptide sequence.


