SKF 77434 hydrobromideSelective D1-like partial agonist CAS# 300561-58-4 |
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- SB 239063
Catalog No.:BCC1923
CAS No.:193551-21-2
- SD-06
Catalog No.:BCC1937
CAS No.:271576-80-8
- BIRB 796 (Doramapimod)
Catalog No.:BCC2535
CAS No.:285983-48-4
- LY2228820
Catalog No.:BCC2528
CAS No.:862507-23-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 300561-58-4 | SDF | Download SDF |
| PubChem ID | 11957703 | Appearance | Powder |
| Formula | C19H22BrNO2 | M.Wt | 376.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in water and to 100 mM in DMSO | ||
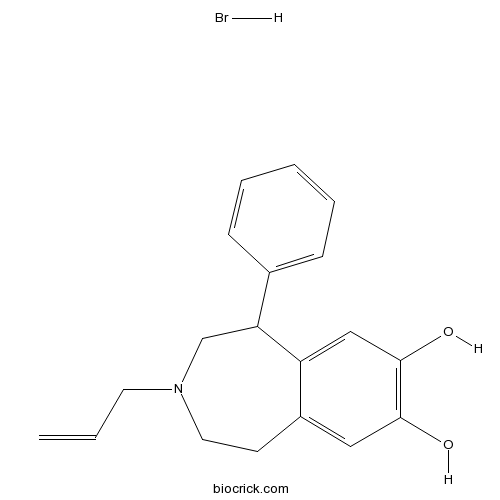
| Chemical Name | 5-phenyl-3-prop-2-enyl-1,2,4,5-tetrahydro-3-benzazepine-7,8-diol;hydrobromide | ||
| SMILES | C=CCN1CCC2=CC(=C(C=C2C(C1)C3=CC=CC=C3)O)O.Br | ||
| Standard InChIKey | JWQRAXTWDYUBFI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H21NO2.BrH/c1-2-9-20-10-8-15-11-18(21)19(22)12-16(15)17(13-20)14-6-4-3-5-7-14;/h2-7,11-12,17,21-22H,1,8-10,13H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective dopamine D1-like receptor partial agonist (IC50 values are 19.7 and 2425 nM for binding to D1-like and D2-like receptors respectively). Centrally active following systemic administration in vivo. |

SKF 77434 hydrobromide Dilution Calculator

SKF 77434 hydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6575 mL | 13.2876 mL | 26.5752 mL | 53.1505 mL | 66.4381 mL |
| 5 mM | 0.5315 mL | 2.6575 mL | 5.315 mL | 10.6301 mL | 13.2876 mL |
| 10 mM | 0.2658 mL | 1.3288 mL | 2.6575 mL | 5.315 mL | 6.6438 mL |
| 50 mM | 0.0532 mL | 0.2658 mL | 0.5315 mL | 1.063 mL | 1.3288 mL |
| 100 mM | 0.0266 mL | 0.1329 mL | 0.2658 mL | 0.5315 mL | 0.6644 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dehydrocorydalin
Catalog No.:BCN2474
CAS No.:30045-16-0
- Boc-Asn-ol
Catalog No.:BCC2587
CAS No.:30044-67-8
- Ro 28-1675
Catalog No.:BCC4124
CAS No.:300353-13-3
- Diphyllin O-glucoside
Catalog No.:BCN8065
CAS No.:30021-77-3
- HEAT hydrochloride
Catalog No.:BCC6683
CAS No.:30007-39-7
- Isoline
Catalog No.:BCN2063
CAS No.:30000-36-3
- Hypotaurine
Catalog No.:BCN1749
CAS No.:300-84-5
- H-Tyr(3,5-I2)-OH
Catalog No.:BCC3263
CAS No.:300-39-0
- Arecoline hydrobromide
Catalog No.:BCN2913
CAS No.:300-08-3
- YM 202074
Catalog No.:BCC7682
CAS No.:299900-84-8
- 1,3,5-Trihydroxy-4-(3-hydroxy-3-methylbutyl)xanthone
Catalog No.:BCN1462
CAS No.:299895-11-7
- NKY 80
Catalog No.:BCC8003
CAS No.:299442-43-6
- HMBA Linker
Catalog No.:BCC2831
CAS No.:3006-96-0
- BMS 309403
Catalog No.:BCC8046
CAS No.:300657-03-8
- TG003
Catalog No.:BCC4416
CAS No.:300801-52-9
- RS 504393
Catalog No.:BCC1910
CAS No.:300816-15-3
- Ciluprevir (BILN-2061)
Catalog No.:BCC1482
CAS No.:300832-84-2
- GSA 10
Catalog No.:BCC6329
CAS No.:300833-95-8
- 4-CMTB
Catalog No.:BCC6250
CAS No.:300851-67-6
- Methyl Linolenate
Catalog No.:BCN8318
CAS No.:301-00-8
- Oleamide
Catalog No.:BCC6827
CAS No.:301-02-0
- Robinin
Catalog No.:BCN5208
CAS No.:301-19-9
- Malvidin-3-O-galactoside chloride
Catalog No.:BCN3030
CAS No.:30113-37-2
- Tianeptine sodium
Catalog No.:BCC2506
CAS No.:30123-17-2
Dopamine D1 receptor family agonists, SK&F38393, SK&F77434, and SK&F82958, differentially affect locomotor activities in rats.[Pubmed:7903456]
Pharmacol Biochem Behav. 1993 Oct;46(2):269-74.
Dopamine D1 receptor family agonists, 2,3,4,5,-tetrahydro-7,8-dihydroxy-1phenyl-1H-3-benzazepine (SK&F38393), 3-allyl-2,3,4,5-tetrahydro-7,8-dihydroxy-1-phenyl-1H-3-benzazepine (SK&F77434), and 3-allyl-6-chloro-2,3,4,5-tetrahydro-7,8-dihydroxy-1-phenyl-1H-3-benzazep ine (SK&F82958), were compared for their behavioral effects on horizontal movement time, rearing time, stereotypy time, and thigmotaxis time. All agonists resulted in biphasic effects with attenuation followed by potentiation (0.01-10.0 mg/kg, SC). While SK&F38393 did not potentiate horizontal movement and rearing times, and had minor effects on thigmotaxis, SK&F77434 and SK&F82958 potentiated horizontal movement and rearing behaviors and attenuated thigmotaxis. The results were discussed in terms of the binding characteristics and current receptor theory.
Stereoisomeric probes for the D1 dopamine receptor: synthesis and characterization of R-(+) and S-(-) enantiomers of 3-allyl-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine and its 6-bromo analogue.[Pubmed:1533424]
J Med Chem. 1992 Apr 17;35(8):1466-71.
Substituted 1-phenyl-3-benzazepines (e.g., SKF 38393 and fenoldopam) exhibit stereoselectivity in moderately high-affinity binding to and partial agonist activation of D1 dopamine receptors. The 3-allyl (APB) and the 3-allyl-6-chloro (6-Cl-APB) analogues of SKF 38393 are reported to have higher affinity and selectivity for the D1 DA receptor and higher in vivo central neuropharmacologic activity than SKF 38393. We recently reported the corresponding 3-allyl-6-bromo analogue (6-Br-APB) also to be a high-affinity D1 agonist. We now describe the synthesis and characterization of the R-(+) and S-(-) enantiomers of both APB and 6-Br-APB and their comparison with corresponding enantiomers of SKF 38393 with respect to D1 receptor binding affinity and D1 and D2 selectivity. The R-(+) enantiomers of both novel substituted 1-phenyl-3-benzazepines bound to the D1 receptor sites in rat forebrain tissue with much higher affinity and selectivity than their S-(-) antipodes. R-(+)-3-Allyl-6-bromo-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3- benzazepine [(R)-(+)-6-Br-APB, 18] exhibits the highest affinity of the reported 1-phenyl-3-benzazepine D1 agonists.
Activation of the 5-HT1C receptor expressed in Xenopus oocytes by the benzazepines SCH 23390 and SKF 38393.[Pubmed:1687364]
Br J Pharmacol. 1991 Dec;104(4):1038-44.
1. A cloned 5-HT1C receptor expressed in Xenopus laevis oocytes was used to characterize the action of four dopamine D1-selective benzazepines at the 5-HT1C receptor. Additionally, the apparent binding of the D1-selective benzazepines to 5-HT1C receptors was measured in the choroid plexus of the pig. 2. In voltage-clamped oocytes expressing the cloned 5-HT1C receptor, 5-hydroxytryptamine (5-HT) elicited a characteristic inward current response with an EC50 of 13 nM. SCH 23390 acted as a stereoselective agonist (or partial agonist) with an EC50 of about 550 nM. SKF 38393 (1 microM-1 mM), SKF 77434 (100 microM), and SKF 82958 (100 microM) also acted as agonists (or partial agonists) at the cloned 5-HT1C receptor. SKF 38393 was not stereoselective at the 5-HT1C receptor. 3. The response to SCH 23390 activated slowly and, although the response contained many oscillations characteristic of the activation of the phosphatidylinositol signal transduction system, SCH 23390 rarely elicited the rapid spike-like response seen routinely in response to 5-HT. However, the responses to SKF 38393, SKF 77434, and SKF 82958 were identical in appearance to the response to 5-HT, except that the responses to the benzazepines were smaller. These comparisons were made by applying both a benzazepine and 5-HT to each individual oocyte expressing the cloned 5-HT1C receptor. 4. Consistent with the responses measured in oocytes, SCH 23390 bound stereoselectively to 5-HT1C receptors in the choroid plexus of the pig (Ki = 6.3 nM), and SKF 38393 bound non-stereoselectively with lower affinity (Ki = 2.0-2.2 microM).5. It is concluded that while these benzazepines demonstrate selectivity for the dopamine D1 receptor, they also can act as agonists or partial agonists at the 5-HT1c receptor in situ and as expressed in Xenopus oocytes. The oocyte expression system is useful for studies of the functional pharmacology of these 5-HTic receptors. Information about the pharmacological actions and variations in stereoselectivity among dopamine and 5-HT receptors should be of interest in modelling the interactions of ligands with these G-protein coupled receptors, and in the testing of such models through receptor mutagenesis.


