Sanggenon OCAS# 101664-32-8 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 101664-32-8 | SDF | File under preparation. |
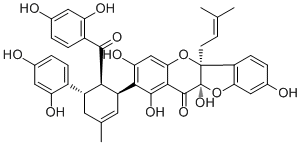
| PubChem ID | N/A | Appearance | Yellow powder |
| Formula | C40H36O12 | M.Wt | 708.76 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Sanggenon O Dilution Calculator

Sanggenon O Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4109 mL | 7.0546 mL | 14.1091 mL | 28.2183 mL | 35.2729 mL |
| 5 mM | 0.2822 mL | 1.4109 mL | 2.8218 mL | 5.6437 mL | 7.0546 mL |
| 10 mM | 0.1411 mL | 0.7055 mL | 1.4109 mL | 2.8218 mL | 3.5273 mL |
| 50 mM | 0.0282 mL | 0.1411 mL | 0.2822 mL | 0.5644 mL | 0.7055 mL |
| 100 mM | 0.0141 mL | 0.0705 mL | 0.1411 mL | 0.2822 mL | 0.3527 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sibiriquinone B
Catalog No.:BCX0259
CAS No.:723300-09-2
- Suffruticosol B
Catalog No.:BCX0258
CAS No.:220936-87-8
- Arucadiol
Catalog No.:BCX0257
CAS No.:105037-85-2
- (E)-α-Atlantone
Catalog No.:BCX0256
CAS No.:26294-59-7
- (E)-4-(4-Hydroxyphenyl)but-3-en-2-one
Catalog No.:BCX0255
CAS No.:22214-30-8
- Dulcisxanthone B
Catalog No.:BCX0254
CAS No.:869669-62-5
- Chasmanthin
Catalog No.:BCX0253
CAS No.:20379-19-5
- Sanggenon B
Catalog No.:BCX0252
CAS No.:81381-67-1
- Kaempferol 3-O-(2''-O-glucosyl)rutinoside
Catalog No.:BCX0251
CAS No.:55696-58-7
- Dehydrozingerone
Catalog No.:BCX0250
CAS No.:1080-12-2
- (2S,3S)-Pteroside C
Catalog No.:BCX0249
CAS No.:98855-62-0
- (2R,3S)-Pteroside C
Catalog No.:BCX0248
CAS No.:68399-16-6
- (2S,3S)-Pterosin S 14-O-glucoside
Catalog No.:BCX0261
CAS No.:62043-50-9
- Pruniflorone R
Catalog No.:BCX0262
CAS No.:1238102-65-2
- Assiguxanthone B
Catalog No.:BCX0263
CAS No.:197447-29-3
- N-(3-(4-Fluorophenyl)propyl)-7-hydroxycoumarin-3-carboxamide
Catalog No.:BCX0264
CAS No.:2136579-33-2
- Suffruticosol D
Catalog No.:BCX0265
CAS No.:1261292-11-8
- Isogarcinol
Catalog No.:BCX0266
CAS No.:71117-97-0
- (E)-Eucomin
Catalog No.:BCX0267
CAS No.:17934-08-6
- Marsdenoside I
Catalog No.:BCX0268
CAS No.:872993-75-4
- Daphnetin 8-O-glucoside
Catalog No.:BCX0269
CAS No.:20853-56-9
- Euryanoside
Catalog No.:BCX0270
CAS No.:125300-52-9
- Suffruticosol A
Catalog No.:BCX0271
CAS No.:220936-82-3
- 6α-Hydroxystigmast-4-en-3-one
Catalog No.:BCX0272
CAS No.:75686-40-7
Investigating into the molecular interactions of flavonoids targeting NS2B-NS3 protease from ZIKA virus through in-silico approaches.[Pubmed:31920173]
J Biomol Struct Dyn. 2021 Jan;39(1):272-284.
Zika virus (ZIKV), belongs to the flavivirus genus and Flaviviridae family that associated with serious diseased conditions like microcephaly and other neurological disorders (Guillan-Barre syndrome). As there is no vaccine or therapies available against ZIKV to date. Hence, it is an unmet need to find potential drug candidates and target sites against Zika virus infection. NS2B-NS3 protease making an attractive target for therapeutic intervention in ZIKV infections because of its critical role in hydrolysis of a single polyprotein encoded by Zika virus. Recently, there are some experimental evidence about the flavonoids as Zika virus NS2B-NS3 protease inhibitors. However, molecular interaction between protease complex and inhibitors at atomic levels has not been explored. Here, we have taken the experimentally validated thirty-eight flavonoids inhibitors against NS2B-NS3 protease to examine the molecular interaction using molecular docking and molecular dynamics simulations. We found out few flavonoids such as EGCG and its two derivatives, isoquercetin, rutin and Sanggenon O showing interaction with catalytic triad (His51, Asp75, and Ser135) of the active site of NS2B-NS3 protease and found to be stable throughout the simulation. Therefore it is evident that interaction with the catalytic triad playing a vital role in the inhibition of the enzyme activity as a result inhibition of the virus propagation. However these compounds can be explored further for understanding the mechanism of action of these compounds targeting NS2B-NS3 protease for inhibition of Zika virus.
Sanggenon O induced apoptosis of A549 cells is counterbalanced by protective autophagy.[Pubmed:30953888]
Bioorg Chem. 2019 Jun;87:688-698.
Sanggenon O (SO) is a Diels-Alder type adduct extracted fromMorus alba, which has been used for its anti-inflammatory action in the Oriental medicine. However, whether it has regulatory effect on human cancer cell proliferation and what the underlying mechanism remains unknown. Here, we found that SO could significantly inhibit the growth and proliferation of A549 cells and induce its pro-apoptotic action through a caspase-dependent pathway. It could also impair the mitochondria which can be reflected by mitochondrial membrane permeabilization. Besides, SQSTM1 up-regulation and autophagic flux measurement demonstrated that exposure to SO led to autophagosome accumulation, which plays a protective role in SO-treated cells. In addition, knocking down of LC3B increased SO triggered apoptotic cell rates. These results indicated that SO has great potential as a promising candidate combined with autophagy inhibitor for the treatment of NSCLC. In conclusion, our results identified a novel mechanism by which SO exerts potent anticancer activity.
Isoprenylated phenolic compounds with tyrosinase inhibition from Morus nigra.[Pubmed:29191050]
J Asian Nat Prod Res. 2018 May;20(5):488-493.
A new isoprenylated sanggenon-type flavanone, nigrasin K (1), together with three known analogs (2-4) and five known Diels-Alder adducts (5-9), were isolated from the twigs of Morus nigra. Their structures were elucidated by spectroscopic methods. Sanggenon M (2), chalcomoracin (5), sorocein H (6), kuwanon J (7), sanggenon C (8), and Sanggenon O (9) showed significant inhibitory effects on mushroom tyrosinase.
Molecular Docking Based Screening of Plant Flavonoids as Dengue NS1 Inhibitors.[Pubmed:25187688]
Bioinformation. 2014 Jul 22;10(7):460-5.
Dengue infection has turned into a serious health concern globally due to its high morbidity rate and a high possibility of increase in its mortality rate on the account of unavailability of any proper treatment for severe dengue infection. The situation demands an urgent development of efficient and practicable treatment to deal with Dengue virus (DENV). Flavonoids, a class of phytochemicals present in medicinal plants, possess anti-viral activity and can be strong drug candidates against viruses. NS1 glycoprotein of Dengue virus is involved in its RNA replication and can be a strong target for screening of drugs against this virus. Current study focuses on the identification of flavonoids which can block Asn-130 glycosylation site of Dengue virus NS1 to inhibit viral replication as glycosylation of NS1 is required for its biological functioning. Molecular docking approach was used in this study and the results revealed that flavonoids have strong potential interactions with active site of NS1. Six flavonoids (Deoxycalyxin A; 3,5,7,3',4'-pentahydroxyflavonol-3-O-beta-D-galactopyranoside; (3R)-3',8-Dihydroxyvestitol; Sanggenon O; Epigallocatechin gallate; Chamaejasmin) blocked the Asn-130 glycosylation site of NS1 and could be able to inhibit the viral replication. It can be concluded from this study that these flavonoids could serve as antiviral drugs for dengue infections. Further in-vitro analyses are required to confirm their efficacy and to evaluate their drug potency.
Sanggenon C and O inhibit NO production, iNOS expression and NF-kappaB activation in LPS-induced RAW264.7 cells.[Pubmed:21612567]
Immunopharmacol Immunotoxicol. 2012 Feb;34(1):84-8.
OBJECTIVE: The NO production through the iNOS induction by activation of nuclear factor (NF-kappaB) is known to involve in various inflammatory conditions. Sanggenon C and O, two Diels-Alder type adducts isolated from Morus alba, a plant has been used for the anti-inflammatory purpose in the Oriental medicine, were investigated for their effect on the NO production, iNOS expression and NF-kappaB activity. METHODS: The inhibitory effects of sanggenon C and O on the NF-kappaB activity were investigated in LPS-stimulated RAW264.7 cells by SEAP reporter assay. The regulation of the iNOS expression and IkappaBalpha activation by two compounds was also evaluated by Western blot. RESULTS: Both compounds strongly inhibited NO production and NF-kappaB activation in a dose-dependent manner. The expression of the iNOS protein was also suppressed by treatment of the compounds (10 and 1 microM). Sanggenon O showed stronger inhibition than the diastereomer sanggenon C. Both compounds prevented the phosphorylation and degradation of IkappaBalpha protein. CONCLUSION: We demonstrated that sanggenon C and O inhibited NO production and iNOS expression by suppressing NF-kappaB activity and IkappaBalpha activation.
Diels-Alder type adducts from Morus cathayana.[Pubmed:11454350]
Phytochemistry. 2001 Aug;57(8):1231-5.
Two natural Diels-Alder type adducts, cathayanon A (1) and cathayanon B (2), resembling sanggenon C (4) and O (3), were isolated from the root bark of Morus cathayana. Their structures were elucidated on the basis of spectroscopic evidence. The structure of 1 was confirmed by the results of X-ray crystallographic analysis. The absolute configurations of 1 and 2 were determined as 2S, 3R,14S, 19S, 20R and 2S, 3R, 14R, 19S, 20R, respectively. The absolute configurations at C-2 and C-3 of two other known isomeric Diels-Alder adducts Sanggenon O (3) and C (4) were deduced as 2R, 3S. Pharmacological tests indicated that 1 and 2 exhibited potent activities on the inhibition of HL-60 cell adhesion to BAEC at concentrations of 10(-5) mol l(-1), with inhibitory rates of 44.72 and 39.02%, respectively.


