Suffruticosol BCAS# 220936-87-8 |
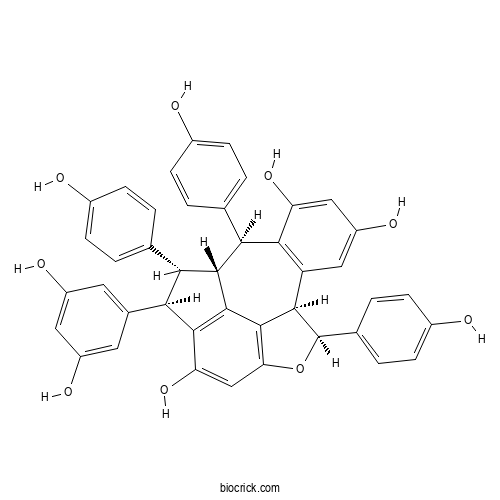
- Suffruticosol A
Catalog No.:BCX0271
CAS No.:220936-82-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 220936-87-8 | SDF | Download SDF |
| PubChem ID | 10652146 | Appearance | Brown powder |
| Formula | C42H32O9 | M.Wt | 680.7 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,2R,3R,9S,10R,17S)-3-(3,5-dihydroxyphenyl)-2,9,17-tris(4-hydroxyphenyl)-8-oxapentacyclo[8.7.2.04,18.07,19.011,16]nonadeca-4(18),5,7(19),11(16),12,14-hexaene-5,13,15-triol | ||
| SMILES | C1=CC(=CC=C1C2C3C(C4=C(C=C(C=C4O)O)C5C(OC6=C5C3=C(C2C7=CC(=CC(=C7)O)O)C(=C6)O)C8=CC=C(C=C8)O)C9=CC=C(C=C9)O)O | ||
| Standard InChIKey | MBGBQHRAJPLAPN-BTJJUUGGSA-N | ||
| Standard InChI | InChI=1S/C42H32O9/c43-23-7-1-19(2-8-23)33-35(22-13-26(46)15-27(47)14-22)38-31(50)18-32-39-37(42(51-32)21-5-11-25(45)12-6-21)29-16-28(48)17-30(49)36(29)34(40(33)41(38)39)20-3-9-24(44)10-4-20/h1-18,33-35,37,40,42-50H/t33-,34-,35-,37+,40+,42+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Suffruticosol B Dilution Calculator

Suffruticosol B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4691 mL | 7.3454 mL | 14.6908 mL | 29.3815 mL | 36.7269 mL |
| 5 mM | 0.2938 mL | 1.4691 mL | 2.9382 mL | 5.8763 mL | 7.3454 mL |
| 10 mM | 0.1469 mL | 0.7345 mL | 1.4691 mL | 2.9382 mL | 3.6727 mL |
| 50 mM | 0.0294 mL | 0.1469 mL | 0.2938 mL | 0.5876 mL | 0.7345 mL |
| 100 mM | 0.0147 mL | 0.0735 mL | 0.1469 mL | 0.2938 mL | 0.3673 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Arucadiol
Catalog No.:BCX0257
CAS No.:105037-85-2
- (E)-α-Atlantone
Catalog No.:BCX0256
CAS No.:26294-59-7
- (E)-4-(4-Hydroxyphenyl)but-3-en-2-one
Catalog No.:BCX0255
CAS No.:22214-30-8
- Dulcisxanthone B
Catalog No.:BCX0254
CAS No.:869669-62-5
- Chasmanthin
Catalog No.:BCX0253
CAS No.:20379-19-5
- Sanggenon B
Catalog No.:BCX0252
CAS No.:81381-67-1
- Kaempferol 3-O-(2''-O-glucosyl)rutinoside
Catalog No.:BCX0251
CAS No.:55696-58-7
- Dehydrozingerone
Catalog No.:BCX0250
CAS No.:1080-12-2
- (2S,3S)-Pteroside C
Catalog No.:BCX0249
CAS No.:98855-62-0
- (2R,3S)-Pteroside C
Catalog No.:BCX0248
CAS No.:68399-16-6
- Pterosin L 2'-O-glucoside
Catalog No.:BCX0247
CAS No.:61102-11-2
- Pyridylpaeoniflorin
Catalog No.:BCX0246
CAS No.:1427054-19-0
- Sibiriquinone B
Catalog No.:BCX0259
CAS No.:723300-09-2
- Sanggenon O
Catalog No.:BCX0260
CAS No.:101664-32-8
- (2S,3S)-Pterosin S 14-O-glucoside
Catalog No.:BCX0261
CAS No.:62043-50-9
- Pruniflorone R
Catalog No.:BCX0262
CAS No.:1238102-65-2
- Assiguxanthone B
Catalog No.:BCX0263
CAS No.:197447-29-3
- N-(3-(4-Fluorophenyl)propyl)-7-hydroxycoumarin-3-carboxamide
Catalog No.:BCX0264
CAS No.:2136579-33-2
- Suffruticosol D
Catalog No.:BCX0265
CAS No.:1261292-11-8
- Isogarcinol
Catalog No.:BCX0266
CAS No.:71117-97-0
- (E)-Eucomin
Catalog No.:BCX0267
CAS No.:17934-08-6
- Marsdenoside I
Catalog No.:BCX0268
CAS No.:872993-75-4
- Daphnetin 8-O-glucoside
Catalog No.:BCX0269
CAS No.:20853-56-9
- Euryanoside
Catalog No.:BCX0270
CAS No.:125300-52-9
Comprehensive comparison of different parts of Paeonia ostii, a food-medicine plant, based on untargeted metabolomics, quantitative analysis, and bioactivity analysis.[Pubmed:37711307]
Front Plant Sci. 2023 Aug 29;14:1243724.
INTRODUCTION: Paeonia ostii T. Hong & J.X. Zhang (s.s.) (Chinese name, Fengdan) is a widely cultivated food-medicine plant in China, in which root bark, seed kernels, and flowers are utilized for their medicinal and edible values. However, other parts of the plant are not used efficiently, in part due to a poor understanding of their chemical composition and potential biological activity. METHODS: Untargeted ultra-performance liquid chromatography-quadrupole time of flight-mass spectrometry (UPLC-Q-TOF-MS) metabolomics was applied to characterize the metabolic profiles of 10 different parts of P. ostii. RESULTS AND DISCUSSION: A total of 160 metabolites were alternatively identified definitely or tentatively, which were significantly different in various plant parts by multivariate statistical analysis. Quantitative analysis showed that underutilized plant parts also contain many active ingredients. Compared with the medicinal part of root bark, the root core part still contains a higher content of paeoniflorin (17.60 +/- 0.06 mg/g) and PGG (15.50 +/- 2.00 mg/g). Petals, as an edible part, contain high levels of quercitrin, and stamens have higher methyl gallate and PGG. Unexpectedly, the ovary has the highest content of methyl gallate and rather high levels of PGG (38.14 +/- 1.27 mg/g), and it also contains surprisingly high concentrations of floralalbiflorin I. Paeoniflorin (38.68 +/- 0.76 mg/g) is the most abundant in leaves, and the content is even higher than in the root bark. Branches are also rich in a variety of catechin derivatives and active ingredients such as hydrolyzable tannins. Seed kernels also contain fairly high levels of paeoniflorin and albiflorin. Fruit shells still contain a variety of components, although not at high levels. Seed coats, as by-products removed from peony seeds before oil extraction, have high contents of stilbenes, such as trans-gnetin H and Suffruticosol B, showing significant potential for exploitation. Except for the seed kernels, extracts obtained from other parts exhibited good antioxidant activity in DPPH, ABTS, and ferric ion reducing antioxidant power (FRAP) assays (0.09-1.52 mmol TE/g). Five compounds (gallic acid, PGG, trans-resveratrol, kaempferol, and quercitrin) were important ingredients that contributed to their antioxidant activities. Furthermore, P. ostii seed cakes were first reported to possess agonistic activity toward CB1/CB2 receptors. This study provides a scientific basis for the further development and utilization of P. ostii plant resources.
Inhibitory Effect of Monoterpenoid Glycosides Extracts from Peony Seed Meal on Streptococcus suis LuxS/AI-2 Quorum Sensing System and Biofilm.[Pubmed:36498098]
Int J Environ Res Public Health. 2022 Nov 30;19(23):16024.
Streptococcus suis LuxS/AI-2 quorum sensing system regulates biofilm formation, resulting in increased pathogenicity and drug resistance, and diminished efficacy of antibiotic treatment. The remaining peony seed cake after oil extraction is rich in monoterpenoid glycosides, which can inhibit the formation of bacterial biofilm. In this study, we investigated the effect of seven major monocomponents (suffruticosol A, Suffruticosol B, suffruticosol C, paeonifloin, albiflorin, trans-epsilon-viniferin, gnetin H) of peony seed meal on minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of S. suis. The results showed that the MICs of the seven single components were all greater than 200 mug/mL, with no significant bacteriostatic and bactericidal advantages. Crystal violet staining and scanning electron microscope observation showed that the seven single components had a certain inhibitory effect on the biofilm formation ability of S. suis at sub-MIC concentration. Among them, the ability of paeoniflorin to inhibit biofilm was significantly higher than that of the other six single components. AI-2 signaling molecules were detected by bioreporter strain Vibrio harvey BB170. The detection results of AI-2 signal molecules found that at 1/2 MIC concentration, paeoniflorin significantly inhibited the production of S. suis AI-2 signal, and the inhibitory effect was better than that of the other six single components. In addition, molecular docking analysis revealed that paeoniflorin had a significant binding activity with LuxS protein compared with the other six single components. The present study provides evidence that paeoniflorin plays a key role in the regulation of the inhibition of S. suis LuxS/AI-2 system and biofilm formation in peony seed meal.
Efficient discovery of potent alpha-glucosidase inhibitors from Paeoniae lactiflora using enzyme-MOF nanocomposites and competitive indicators.[Pubmed:36477546]
Food Funct. 2023 Jan 3;14(1):171-180.
A great deal of attention has been paid to the seeds of Paeoniae lactiflora pall., an underutilized food resource, since its extract exhibits excellent alpha-glucosidase (GAA) inhibitory activity. In the present study, to gain further insight into this plant and find out potent GAA inhibitors, we established a novel ligand fishing strategy by introducing a competitive inhibitor as an indicator. After the successful establishment of this approach was verified by a series of methods, including kinetic assay, fluorescence determination, and HPLC, the newly developed ligand fishing method was applied to acquire potent GAA inhibitors from P. lactiflora seeds. Nine bioactive compounds were captured, and seven of them were identified as suffruticosol A, Suffruticosol B, resveratrol, vitisin E, luteolin, trans-delta-viniferin, and ampelopsin E. The data of their GAA inhibitory activity demonstrated that these constituents were vigorously active against GAA with IC(50) values of 1.67-30.47 muM, while such value of 1-DNJ was 228.77 muM. Among them, vitisin E and ampelopsin E were reported to show such inhibitory activity for the first time. Collectively, our findings provide valuable clues for the further utilization of P. lactiflora seeds as a functional food, and offer a new avenue for acquiring potent inhibitors from natural resources.
Suffruticosol B Is an Osteogenic Inducer through Osteoblast Differentiation, Autophagy, Adhesion, and Migration.[Pubmed:36362346]
Int J Mol Sci. 2022 Nov 4;23(21):13559.
Suffruticosol B (Suf-B) is a stilbene found in Paeonia suffruticosa ANDR., which has been traditionally used in medicine. Stilbenes and their derivatives possess various pharmacological effects, such as anticancer, anti-inflammatory, and anti-osteoporotic activities. This study aimed to explore the bone-forming activities and mechanisms of Suf-B in pre-osteoblasts. Herein, >99.9% pure Suf-B was isolated from P. suffruticosa methanolic extracts. High concentrations of Suf-B were cytotoxic, whereas low concentrations did not affect cytotoxicity in pre-osteoblasts. Under zero levels of cytotoxicity, Suf-B exhibited bone-forming abilities by enhancing alkaline phosphatase enzyme activities, bone matrix calcification, and expression levels with non-collagenous proteins. Suf-B induces intracellular signal transduction, leading to nuclear RUNX2 expression. Suf-B-stimulated differentiation showed increases in autophagy proteins and autophagosomes, as well as enhancement of osteoblast adhesion and transmigration on the ECM. These results indicate that Suf-B has osteogenic qualities related to differentiation, autophagy, adhesion, and migration. This also suggests that Suf-B could have a therapeutic effect as a phytomedicine in skeletal disorders.
Integrating network pharmacology analysis and pharmacodynamic evaluation for exploring the active components and molecular mechanism of moutan seed coat extract to improve cognitive impairment.[Pubmed:36034803]
Front Pharmacol. 2022 Aug 12;13:952876.
Paeonia suffruticosa (Moutan) is a traditional medicinal plant in China. Its seed coat is rich in resveratrol oligomer, especially Suffruticosol B (SB). Previous studies had shown that the seed coat extracts of Paeonia suffruticosa (PSCE) had good cholinesterase inhibitory activity and neuroprotective effect, but the effective dose range was unknown, and the pharmacodynamic components and molecular mechanism of PSCE had not been discussed. The current study aimed to screen the pharmacodynamic components in PSCE and investigate the improvement effect of PSCE and the selected SB on scopolamine-induced cognitive dysfunction in mice and its mechanism. The results of high-throughput sequencing and bioinformatics analysis showed that Suffruticosol B (SB) and trans-gnetin H (GH) might be the main active components of PSCE; PSCE might improve cognitive dysfunction through p53, HIF-1, MAPK, and PI3K-Akt signaling pathways, while SB and GH might improve cognitive dysfunction through HIF-1 signaling pathway. SB and GH had good molecular docking activity with the target of HIF-1 signaling pathway. The pharmacodynamic activities of PSCE and SB were further verified by behavioral experiments. PSCE and SB could improve the recognition ability of familiar and new objects and shorten the escape latency in the Morris Water Maze test (PSCE 120 mg∙kg-1, p < 0.05; SB 60 mg∙kg-1, p < 0.01); PSCE and SB could increase Ach and GSH levels, enhance the activities of ChAT, SOD and CAT, decrease the levels of IL-1beta, IL-6, and TNF-alpha, and decrease the activity of AChE. In conclusion, the results indicated that PSCE might exert pharmacodynamic activity through multiple components, targets, and pathways, and SB and GH might be the main active components of PSCE. PSCE and SB might improve cognitive dysfunction by regulating cholinergic, antioxidant, and anti-inflammatory effects. These results indicated that PSCE and SB might be potential anti-AD drug candidates, providing a scientific basis for the development and utilization of Moutan bark.
Ultrasonic-assisted extraction and adsorption separation: Large-scale preparation of trans-epsilon-Viniferin, suffruficosol B and trans-Gnetin H for the first time.[Pubmed:35995022]
Ultrason Sonochem. 2022 Sep;89:106123.
In this study, a Standard Operating Procedure (SOP) for the large-scale extraction, enrichment, and separation of Suffruticosol B (SB), trans-epsilon-Viniferin (TV), trans-gnetin H (TG) from oil tree peony seeds shell (PSS) was successfully constructed. The ultrasonic-assisted extraction (UAE), macroporous adsorption resin (MAR), and column chromatography (CC) were employed to extract, enrich and separate SB, TV and TG from PSS, and the conditions were optimized. The results implied that SB (1.6937 g), TV (0.5884 g) and TG (3.8786 g) with the purity of 99.67 %, 99.32 % and 98.54 %, respectively, were obtained after the extraction, enrichment and separation. The total yields of the SB, TV and TG were 0.61 mg/g, 0.02 mg/g and 6.64 mg/g with the total extraction rates at 70.55 %, 69.77 % and 78.36 %, respectively. This is the first report on the large-scale extraction, enrichment and separation of oligostilbenes. The SOP in this paper could produce high purity SB, TV and TG, and provide a new idea for PSS as a new oligostilbene resource. The study expands the new development and research field of PSS and provides theoretical support for the green utilization of oil tree peony.
Phytochemical profiles and the hypoglycemic effects of tree peony seed coats.[Pubmed:34739020]
Food Funct. 2021 Nov 29;12(23):11777-11789.
As emerging woody oil crops, the tree peony seeds recently have been attracting great attention for their metabolites and bioactivities. In this research, the phytochemical profiles of the seed coats of tree peonies from different production regions were investigated systematically. Twelve phytochemicals were separated and prepared, mainly belonging to stilbenes. A great variation in stilbene content was detected in the three Paeonia plants, and Paeonia ostii seed coats (POSC) had significantly higher contents of the stilbene compounds than other species. There were nineteen significant correlations between ecogeographical factors and the predominant compounds. A clear discrimination among the species was observed in their HPLC fingerprint and chemometric analysis. Furthermore, POSC extracts could significantly reduce the starch mediated PBG (postprandial blood glucose) levels in normal/diabetic mice. Meanwhile, in vitro enzyme tests revealed that the predominant compounds, Suffruticosol B and ampelopsin D, could effectively and competitively inhibit alpha-glucosidase, indicating that POSC could be a natural source of hypoglycemics in the food and drug fields.
[Simultaneous rapid detection of ten stilbenes in serum of mice by UPLC-MS/MS].[Pubmed:32495569]
Zhongguo Zhong Yao Za Zhi. 2020 May;45(9):2180-2185.
Stilbenes is a class of natural polyphenols with 1,2-diphenylethylene as the skeleton structure which have structural and active diversity. However, there are fewer studies on their metabolic process, which limits the in-depth research and development of such components. An UPLC-MS/MS method simultaneously determining contents of ten stilbenes was firstly established in this study and applied to study the ten stilbenes of peony seed coats in the serum of C57 mice.Piceatannol was the internal standard, and methanol was used for protein precipitation, UPLC-MS/MS with negative ion mode was used for analysis, and the method was validated.The serum samples were collected and detected after mice being oral administered with 800 mg.kg~(-1) peony seed coat extracts for 8 weeks. The results showed that suffruticosol A, Suffruticosol B, suffruticosol C, trans-epsilon-viniferin, cis-gnetin H, trans-suffruticosol D and trans-gnetin H were detected in serum samples, and the highest is suffruticosol A. The method is simple and quick with high specificity and sensitivity, and it is suitable for quantitative determination of ten stilbenes in the serum of mice.
[Determination of ten stilbenes and their antioxidant activity of peony seed coat, seed kernel and seed coat extracts].[Pubmed:28875674]
Zhongguo Zhong Yao Za Zhi. 2016 Mar;41(6):1081-1086.
The seed of tree peony and herbaceous peony contained a variety of stilbenes which possess many pharmacological activities, such as antitumor, anti-inflammatory, allergy and neuraminidase inhibition. In order to develop and use peony seed resources, a simple and sensitive HPLC-DAD was developed for simultaneous determination of 10 stilbenes in peony samples, i.e.,suffruticosol A,Suffruticosol B,suffruticosol C,trans-resveratrol,cis-epsilon-viniferin,trans-epsilon-viniferin,cis-suffruticosol D,cis-gnetin H,trans-suffruticosol D and trans-gnetin H. At the same time, the scavenging activity of DPPH free radicals was employed to evaluate their antioxidant effect. The results showed that the 10 stilbenes are mainly present in peony seed coat (total content of more than 16.7%) compared to peony seed kernel (total content less than 0.3%), and can be enriched in the extract of peony seed coat (total content of more than 75%) The extract of peony seed coat and 10 stilbenes exhibited significant antioxidant properties. This work provides a foundation for comprehensive utilization of the tree peony and herbaceous peony seed resources.
Resveratrol trimers from seed cake of Paeonia rockii.[Pubmed:25532833]
Molecules. 2014 Nov 26;19(12):19549-56.
In the course of screening natural products for antibacterial activities, a total acetone extract of the seed cake of Paeonia rockii showed significant effects against bacterial strains. Bioactivity-guided fractionation of the EtOAc-soluble fraction of the total acetone extract resulted in the isolation and identification of five resveratrol trimers, including rockiiol C (1), gnetin H (2), suffruticosol A (3), Suffruticosol B (4) and suffruticosol C (5). The relative configuration of these compounds was elucidated mainly by comprehensive 1D and 2D-NMR experiments. Compound 1 was a new compound. All isolated compounds exhibited strong antibacterial activities against Gram-positive bacteria.
Resveratrol oligomers inhibit biofilm formation of Escherichia coli O157:H7 and Pseudomonas aeruginosa.[Pubmed:24456071]
J Nat Prod. 2014 Jan 24;77(1):168-72.
Biofilm formation is closely related to bacterial infection and is also a mechanism of antimicrobial resistance. Hence, the antibiofilm approach provides an alternative to an antibiotic strategy. In this study, the antibiofilm activities of resveratrol (1) and five of its oligomers, namely, epsilon-viniferin (2), suffruticosol A (3), Suffruticosol B (4), vitisin A (5), and vitisin B (6), were investigated against enterohemorrhagic Escherichia coli O157:H7 and Pseudomonas aeruginosa PA14. Vitisin B (6), a stilbenoid tetramer, was found to inhibit biofilm formation by the two bacteria the most effectively and at 5 mug/mL inhibited E. coli O157:H7 biofilm formation by more than 90%.
Profiling of neuraminidase inhibitory polyphenols from the seeds of Paeonia lactiflora.[Pubmed:23313796]
Food Chem Toxicol. 2013 May;55:144-9.
Bacterial neuraminidase (NA) is a lynch pin enzyme in the formation of biofilms. Thus NA continues to be one of the key enzymes targeted by bacterial infection. The purpose of this manuscript is to communicate four new naturally derived inhibitors of neuraminidase (IC50s 3.7-24.4muM). All these active species (1-4) contained a resveratrol chemotype, however resveratrol itself was inactive (IC50>100muM). 1-4 were isolated from the 60% aqueous ethanol extract of seeds of Paeonia lactiflora, which exhibited potent neuraminidase inhibition. Purification of the extracts yielded four chiral polyphenols, suffruticosol A (1), Suffruticosol B (2), trans-epsilon-viniferin (3), and trans-gnetin H (4). Mechanistic analysis of 1-4's inhibition showed that they were all reversible, noncompetitive inhibitors. Trans-epsilon-viniferin (3) underwent trans-cis isomerization, which led to a reduction in inhibition potency. This correlates with the fact that the cis-isomer is a weaker inhibitor of neuraminidase than the trans-isomer. Importantly, significantly different optical rotations ([alpha]D) compared to previous reports were found for suffruticosols A (+95 vs -34) and B (+136 vs +13). These two species are the most important standard metabolites in the whole paeoniaceae family and therefore correction of this error is important.
Three new oligostilbenes from the seeds of Paeonia suffruticosa.[Pubmed:20522997]
Chem Pharm Bull (Tokyo). 2010 Jun;58(6):843-7.
Three new oligostilbenes, trans-suffruticosol D (1), cis-suffruticosol D (2), and cis-gnetin H (7), were isolated along with the eight known stilbenes, trans-resveratrol (3), trans-epsilon-viniferin (4), cis-epsilon-viniferin (5), gnetin H (6), suffruticosol A (8), Suffruticosol B (9), suffruticosol C (10), and cis-ampelopsin E (11) from the seeds of Paeonia suffruticosa. Compounds 3-6 were isolated for the first time from this plant species, and compound 11 was isolated for the first time from the genus Paeonia. The structures of the new compounds were elucidated based on spectral analyses, including 1D and 2D NMR experiments. The absolute configuration of compound 1 was determined by quantum chemical calculation of the electronic circular dichroism and comparison with the experimental circular dichroism spectrum.


