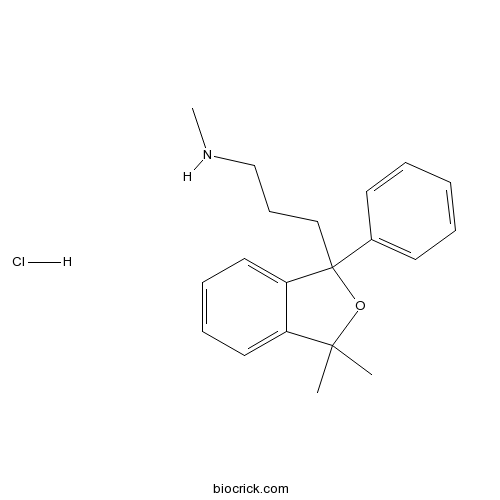
Talopram hydrochloridePotent, selective inhibitor of noradrenalin transporters CAS# 7013-41-4 |
- SB-222200
Catalog No.:BCC1926
CAS No.:174635-69-9
- Talnetant hydrochloride
Catalog No.:BCC1982
CAS No.:204519-66-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 7013-41-4 | SDF | Download SDF |
| PubChem ID | 71176 | Appearance | Powder |
| Formula | C20H26ClNO | M.Wt | 331.88 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Lu 3-010 | ||
| Solubility | Soluble to 50 mM in water and to 100 mM in DMSO | ||
| Chemical Name | 3-(3,3-dimethyl-1-phenyl-2-benzofuran-1-yl)-N-methylpropan-1-amine;hydrochloride | ||
| SMILES | CC1(C2=CC=CC=C2C(O1)(CCCNC)C3=CC=CC=C3)C.Cl | ||
| Standard InChIKey | JZXJIRQPHHWYGC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H25NO.ClH/c1-19(2)17-12-7-8-13-18(17)20(22-19,14-9-15-21-3)16-10-5-4-6-11-16;/h4-8,10-13,21H,9,14-15H2,1-3H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, selective inhibitor of the noradrenalin transporter (NET) (IC50 = 2.9 nM). Exhibits selectivity for NET against SERT (5-HT transporters) and DAT (dopamine transporters). Displays a similar structure but different pharmacological profile to citalopram. |

Talopram hydrochloride Dilution Calculator

Talopram hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0131 mL | 15.0657 mL | 30.1314 mL | 60.2627 mL | 75.3284 mL |
| 5 mM | 0.6026 mL | 3.0131 mL | 6.0263 mL | 12.0525 mL | 15.0657 mL |
| 10 mM | 0.3013 mL | 1.5066 mL | 3.0131 mL | 6.0263 mL | 7.5328 mL |
| 50 mM | 0.0603 mL | 0.3013 mL | 0.6026 mL | 1.2053 mL | 1.5066 mL |
| 100 mM | 0.0301 mL | 0.1507 mL | 0.3013 mL | 0.6026 mL | 0.7533 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- DGAT-1 inhibitor
Catalog No.:BCC1529
CAS No.:701232-20-4
- BMS-626529
Catalog No.:BCC1427
CAS No.:701213-36-7
- 5-Pentacosylresorcinol
Catalog No.:BCN4263
CAS No.:70110-61-1
- 5-Tricosyl-1,3-benzenediol
Catalog No.:BCN4262
CAS No.:70110-60-0
- 5-Heneicosylresorcinol
Catalog No.:BCN7630
CAS No.:70110-59-7
- TCN 237 dihydrochloride
Catalog No.:BCC6111
CAS No.:700878-19-9
- LY2157299
Catalog No.:BCC3709
CAS No.:700874-72-2
- LY2109761
Catalog No.:BCC3806
CAS No.:700874-71-1
- Acronycine
Catalog No.:BCC8114
CAS No.:7008-42-6
- 4,10-Aromadendranediol
Catalog No.:BCN4261
CAS No.:70051-38-6
- LPYFD-NH2
Catalog No.:BCC6113
CAS No.:700361-48-4
- Rivularin
Catalog No.:BCN3189
CAS No.:70028-59-0
- Liquiritic acid
Catalog No.:BCN8332
CAS No.:10379-72-3
- Isotetrandrine N-2'-oxide
Catalog No.:BCN4264
CAS No.:70191-83-2
- Papain Inhibitor
Catalog No.:BCC1024
CAS No.:70195-20-9
- Taranabant
Catalog No.:BCC1985
CAS No.:701977-09-5
- 3-Aminoadamantan-1-ol
Catalog No.:BCC8618
CAS No.:702-82-9
- 8alpha-Hydroxy-alpha-gurjunene
Catalog No.:BCN4265
CAS No.:70206-70-1
- (±)-Lauroylcarnitine chloride
Catalog No.:BCC6690
CAS No.:7023-03-2
- Physalin H
Catalog No.:BCN7917
CAS No.:70241-09-7
- BX-912
Catalog No.:BCC1250
CAS No.:702674-56-4
- BX795
Catalog No.:BCC3635
CAS No.:702675-74-9
- CP 775146
Catalog No.:BCC7881
CAS No.:702680-17-9
- Ivermectin
Catalog No.:BCC1251
CAS No.:70288-86-7
From the selective serotonin transporter inhibitor citalopram to the selective norepinephrine transporter inhibitor talopram: synthesis and structure-activity relationship studies.[Pubmed:18429609]
J Med Chem. 2008 May 22;51(10):3045-8.
Citalopram and talopram are structurally closely related, but they have very distinct pharmacological profiles as selective inhibitors of the serotonin and norepinephrine transporters, respectively. A systematic structure-activity relationship study was performed, in which each of the four positions distinguishing the two compounds were varied. The inhibitory potencies of the resulting 16 compounds were tested at both serotonin and norepinephrine transporters. This showed that particularly two of the four positions are determinants for the biological activity.
3-Phenyl-1-indanamines. Potential antidepressant activity and potent inhibition of dopamine, norepinephrine, and serotonin uptake.[Pubmed:2999402]
J Med Chem. 1985 Dec;28(12):1817-28.
A series of 3-phenyl-1-indanamines was synthesized and tested for potential antidepressant activity and for inhibition of dopamine (DA), norepinephrine (NE), and serotonin (5-HT) uptake. Trans isomers were generally potent inhibitors of DA, NE, and 5-HT uptake, while cis isomers preferentially inhibited the uptake of 5-HT. The affinity for the DA-uptake site was very dependent on the aromatic substitution pattern where highest potency was found for 3',4'-dichloro substituted compounds (45). This substitution pattern also resulted in high affinity for the NE-and 5-HT-uptake sites, but potent 5-HT-uptake inhibiting activity could also be obtained with other substitution patterns. Only small amines could be accommodated at the 5-HT-uptake site while larger amines such as piperazine could be accommodated both at the DA-and NE-uptake sites. The observed structure-activity relationships were explained from the results of superimpositions of a trans (45) and cis (72) isomer with 5-HT and DA, respectively, in relation to a proposed three-point binding of the uptake inhibitors at the uptake sites. Finally, comparison of the structures of the 3-phenyl-1-indanamines with other newer bicyclic catecholamine- and/or serotonin-uptake inhibitors revealed common structural elements important for potent DA-, NE-, and/or 5-HT-uptake inhibition.


