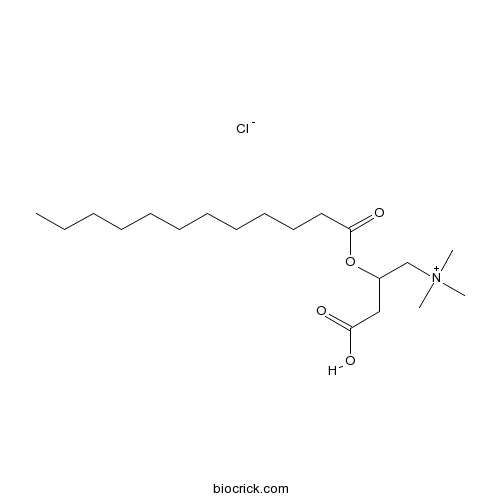
(±)-Lauroylcarnitine chlorideCAS# 7023-03-2 |
- Alvespimycin
Catalog No.:BCC1346
CAS No.:467214-20-6
- 17-AAG (KOS953)
Catalog No.:BCC2121
CAS No.:75747-14-7
- Retaspimycin
Catalog No.:BCC1889
CAS No.:857402-23-4
- PU-H71
Catalog No.:BCC1872
CAS No.:873436-91-0
- 17-AAG Hydrochloride
Catalog No.:BCC1297
CAS No.:911710-03-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 7023-03-2 | SDF | Download SDF |
| PubChem ID | 177509 | Appearance | Powder |
| Formula | C19H38NO4Cl | M.Wt | 379.97 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | (3-carboxy-2-dodecanoyloxypropyl)-trimethylazanium;chloride | ||
| SMILES | [Cl-].CCCCCCCCCCCC(=O)OC(CC(O)=O)C[N+](C)(C)C | ||
| Standard InChIKey | PDBBUDRTWRVCFN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H37NO4.ClH/c1-5-6-7-8-9-10-11-12-13-14-19(23)24-17(15-18(21)22)16-20(2,3)4;/h17H,5-16H2,1-4H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Homolog of acetylcarnitine chloride. Acylcarnitines are important intermediates in lipid metabolism. |

(±)-Lauroylcarnitine chloride Dilution Calculator

(±)-Lauroylcarnitine chloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6318 mL | 13.1589 mL | 26.3179 mL | 52.6357 mL | 65.7947 mL |
| 5 mM | 0.5264 mL | 2.6318 mL | 5.2636 mL | 10.5271 mL | 13.1589 mL |
| 10 mM | 0.2632 mL | 1.3159 mL | 2.6318 mL | 5.2636 mL | 6.5795 mL |
| 50 mM | 0.0526 mL | 0.2632 mL | 0.5264 mL | 1.0527 mL | 1.3159 mL |
| 100 mM | 0.0263 mL | 0.1316 mL | 0.2632 mL | 0.5264 mL | 0.6579 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 8alpha-Hydroxy-alpha-gurjunene
Catalog No.:BCN4265
CAS No.:70206-70-1
- 3-Aminoadamantan-1-ol
Catalog No.:BCC8618
CAS No.:702-82-9
- Taranabant
Catalog No.:BCC1985
CAS No.:701977-09-5
- Papain Inhibitor
Catalog No.:BCC1024
CAS No.:70195-20-9
- Isotetrandrine N-2'-oxide
Catalog No.:BCN4264
CAS No.:70191-83-2
- Liquiritic acid
Catalog No.:BCN8332
CAS No.:10379-72-3
- Talopram hydrochloride
Catalog No.:BCC7579
CAS No.:7013-41-4
- DGAT-1 inhibitor
Catalog No.:BCC1529
CAS No.:701232-20-4
- BMS-626529
Catalog No.:BCC1427
CAS No.:701213-36-7
- 5-Pentacosylresorcinol
Catalog No.:BCN4263
CAS No.:70110-61-1
- 5-Tricosyl-1,3-benzenediol
Catalog No.:BCN4262
CAS No.:70110-60-0
- 5-Heneicosylresorcinol
Catalog No.:BCN7630
CAS No.:70110-59-7
- Physalin H
Catalog No.:BCN7917
CAS No.:70241-09-7
- BX-912
Catalog No.:BCC1250
CAS No.:702674-56-4
- BX795
Catalog No.:BCC3635
CAS No.:702675-74-9
- CP 775146
Catalog No.:BCC7881
CAS No.:702680-17-9
- Ivermectin
Catalog No.:BCC1251
CAS No.:70288-86-7
- Fluoroorotic Acid, Ultra Pure
Catalog No.:BCC1209
CAS No.:703-95-7
- Pertussis Toxin
Catalog No.:BCC7565
CAS No.:70323-44-3
- 3-Benzoylthiazolidine-2-thione
Catalog No.:BCC8624
CAS No.:70326-37-3
- Methyl Kakuol
Catalog No.:BCN8243
CAS No.:70342-29-9
- Avobenzone
Catalog No.:BCC4891
CAS No.:70356-09-1
- Sideritoflavone
Catalog No.:BCN6689
CAS No.:70360-12-2
- Lornoxicam
Catalog No.:BCC4425
CAS No.:70374-39-9
Enhancement of nasal salmon calcitonin absorption by lauroylcarnitine chloride in rats.[Pubmed:8860430]
Pharm Res. 1996 May;13(5):739-43.
PURPOSE: We investigated optimum formulation characteristics in the nasal absorption of salmon calcitonin (sCT) by incorporation of acylcarnitines. METHODS: Nasal sCT formulations were administered to anesthetized rats. Plasma calcium level was measured and pharmacological bioavailability (P.bioav) was calculated. RESULTS: Nasal sCT absorption was significantly enhanced by carnitines with acyl groups of 12 or more carbon atoms. Enhancement by lauroylcarnitine chloride (LCC) was observed at its critical micelle concentration and reached a plateau at the concentration of 0.1 percent. Optimal absorption was achieved at a molar ratio of LCC to sCT of 5:1. Enhancement was not influenced by osmolarity and maximum enhancement was obtained at pHs 3.1 and 4.0. CONCLUSIONS: The 12-carbon LCC was the strongest enhancer among acylcarnitines. Micelle formation played a key role in this enhancement effect.
Determination of acylcarnitines in urine of patients with inborn errors of metabolism using high-performance liquid chromatography after derivatization with 4'-bromophenacylbromide.[Pubmed:8222273]
Clin Chim Acta. 1993 Jul 16;216(1-2):53-61.
A high-performance liquid chromatographic method is presented for the determination of urinary acylcarnitines. After solid phase extraction on silica columns the acylcarnitines are converted to 4'-bromophenacyl esters with 4'-bromophenacylbromide in the presence of N,N-diisopropylethylamine. Complete derivatization was achieved at 37 degrees C within 30 min. The 4'-bromophenacyl esters were separated by high-performance liquid chromatography on a Hypersil BDS C8 reversed-phase column with a binary gradient containing varying proportions of acetonitrile, water and 0.1 M triethylamine phosphate buffer. Essentially baseline separation was obtained with a standard mixture containing 4'-bromophenacyl esters of carnitine and synthetic acylcarnitines of increasing chain length ranging from acetyl- to palmitoylcarnitine. The method was used to obtain urinary acylcarnitine profiles from patients with propionic, methylmalonic and isovaleric acidemia and with medium-chain and multiple acyl-CoA dehydrogenase deficiency. Quantification of the acylcarnitines was achieved using undecanoylcarnitine as internal standard.
Molecular basis of mitochondrial fatty acid oxidation defects.[Pubmed:1431593]
J Lipid Res. 1992 Aug;33(8):1099-110.
A dozen separate inherited disorders of mitochondrial fatty acid beta-oxidation have been described in humans. This represents about half of the potential sites for genetic error that can affect this important pathway of energy metabolism. As the characterization of these disorders at the clinical and biochemical levels has progressed rapidly, so has the delineation of the molecular defects that underlie them. The most commonly recognized disorder of beta-oxidation is medium-chain acyl-CoA dehydrogenase deficiency; a striking feature of this disorder is that there is a single point mutation that accounts for 90% of the variant alleles among patients with medium-chain acyl-CoA dehydrogenase deficiency. Molecular defects of other enzymes in the pathway have been identified, and it seems likely that a complete description of these defects at the molecular level is a realistic goal. In basic biological terms, such studies will lead to a better understanding of the genetic control exerted on this pathway. In clinical terms, they will lead to improved understanding of the molecular pathophysiology of these diseases and may well provide the necessary techniques to proceed with the screening of these disorders.
Urinary excretion of l-carnitine and acylcarnitines by patients with disorders of organic acid metabolism: evidence for secondary insufficiency of l-carnitine.[Pubmed:6441143]
Pediatr Res. 1984 Dec;18(12):1325-8.
Concentrations of l-carnitine and acylcarnitines have been determined in urine from patients with disorders of organic acid metabolism associated with an intramitochondrial accumulation of acyl-CoA intermediates. These included propionic acidemia, methylmalonic aciduria, isovaleric acidemia, multicarboxylase deficiency, 3-hydroxy-3-methylglutaric aciduria, methylacetoacetyl-CoA thiolase deficiency, and various dicarboxylic acidurias including glutaric aciduria, medium-chain acyl-CoA dehydrogenase deficiency, and multiple acyl-CoA dehydrogenase deficiency. In all cases, concentrations of acylcarnitines were greatly increased above normal with free carnitine concentrations ranging from undetectable to supranormal values. The ratios of acylcarnitine/carnitine were elevated above the normal value of 2.0 +/- 1.1. l-Carnitine was given to three of these patients; in each case, concentrations of plasma and urine carnitines increased accompanied by a marked increase in concentrations of short-chain acylcarnitines. These acylcarnitines have been examined using fast atom bombardment mass spectrometry in some of these diseases and have been shown to be propionylcarnitine in methylmalonic aciduria and propionic acidemia, isovalerylcarnitine in isovaleric acidemia, and hexanoylcarnitine and octanoylcarnitine in medium-chain acyl-CoA dehydrogenase deficiency. The excretion of these acylcarnitines is compatible with the known accumulation of the corresponding acyl-CoA esters in these diseases. In this group of disorders, the increased acylcarnitine/carnitine ratio in urine and plasma indicates an imbalance of mitochondrial mass action homeostasis and, hence, of acyl-CoA/CoA ratios. Despite naturally occurring attempts to increase endogeneous l-carnitine biosynthesis, there is insufficient carnitine available to restore the mass action ratio as demonstrated by the further increase in acylcarnitine excretion when patients were given oral l-carnitine.(ABSTRACT TRUNCATED AT 250 WORDS)


