Papain InhibitorInhibitor of peptidase activity of Papain CAS# 70195-20-9 |
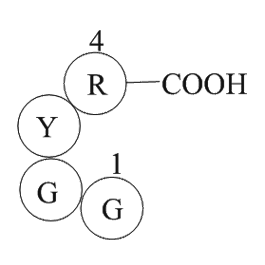
- CUDC-101
Catalog No.:BCC2149
CAS No.:1012054-59-9
- Vorinostat (SAHA, MK0683)
Catalog No.:BCC2145
CAS No.:149647-78-9
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
- PCI-24781 (CRA-024781)
Catalog No.:BCC2155
CAS No.:783355-60-2
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 70195-20-9 | SDF | Download SDF |
| PubChem ID | 125825 | Appearance | Powder |
| Formula | C19H29N7O6 | M.Wt | 451.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GGYR;Gly-Gly-Tyr-Arg;glycyl-glycyl-tyrosyl-arginine | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Sequence | H2N-Gly-Gly-Tyr-Arg-OH | ||
| Chemical Name | (2S)-2-[[(2S)-2-[[2-[(2-aminoacetyl)amino]acetyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-5-(diaminomethylideneamino)pentanoic acid | ||
| SMILES | C1=CC(=CC=C1CC(C(=O)NC(CCCN=C(N)N)C(=O)O)NC(=O)CNC(=O)CN)O | ||
| Standard InChIKey | FJPHHBGPPJXISY-KBPBESRZSA-N | ||
| Standard InChI | InChI=1S/C19H29N7O6/c20-9-15(28)24-10-16(29)25-14(8-11-3-5-12(27)6-4-11)17(30)26-13(18(31)32)2-1-7-23-19(21)22/h3-6,13-14,27H,1-2,7-10,20H2,(H,24,28)(H,25,29)(H,26,30)(H,31,32)(H4,21,22,23)/t13-,14-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Papain Inhibitor Dilution Calculator

Papain Inhibitor Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Papain Inhibitor peptide,(C19H29N7O6),a peptide with the sequence H-Gly-Gly-Tyr-Arg-OH,MW= 451.4,this peptide Inhibits the peptidase activity of Papain. Papain,also known as papaya proteinase I,is a cysteine protease enzyme present in papaya and mountain papaya. The mechanism by which it breaks peptide bonds involves deprotonation of Cys-25 by His-159. Asparagine-175 helps to orient the imidazole ring of His-159 to allow this deprotonation to take place. Cys-25 then performs a nucleophilic attack on the carbonyl carbon of a peptide backbone. This frees the amino terminal of the peptide,and forms a covalent acyl-enzyme intermediate. The enzyme is then deacylated by a water molecule,and releases the carboxy terminal portion of the peptide. In immunology,papain is known to cleave the Fc (crystallisable) portion of immunoglobulins (antibodies) from the Fab (antigen-binding) portion(1). Papain prefers to cleave at: (hydrophobic) -(Arg or Lys)- cleaves here -(not Val). Hydrophobic is Ala,Val,Leu,Ile,Phe,Trp,or Tyr(2). Papain can be used to dissociate cells in the first step of cell culture preparations. It is also used as an ingredient in various enzymatic debriding preparations,notably Accuzyme. These are used in the care of some chronic wounds to clean up dead tissue.Papain can also be found as an ingredient in some toothpastes or mints as teeth-whitener.
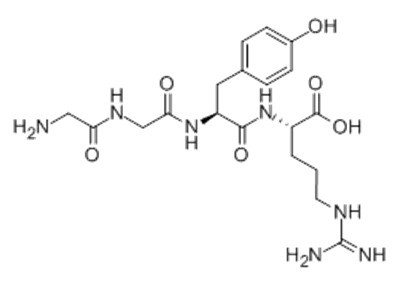
Figure1 Formula of Papain Inhibitor peptide
Figure2 Structure of Papain
Ref:
1. Rawlings ND,Barrett AJ (1994). "Families of cysteine peptidases". Meth. Enzymol. 244: 461–486.
2. Lopes MC,Mascarini RC,da Silva BM,Flório FM,Basting RT (2007). "Effect of a papain-based gel for chemomechanical caries removal on dentin shear bond strength". J Dent Child (Chic) 74 (2): 93–7.
- Isotetrandrine N-2'-oxide
Catalog No.:BCN4264
CAS No.:70191-83-2
- Liquiritic acid
Catalog No.:BCN8332
CAS No.:10379-72-3
- Talopram hydrochloride
Catalog No.:BCC7579
CAS No.:7013-41-4
- DGAT-1 inhibitor
Catalog No.:BCC1529
CAS No.:701232-20-4
- BMS-626529
Catalog No.:BCC1427
CAS No.:701213-36-7
- 5-Pentacosylresorcinol
Catalog No.:BCN4263
CAS No.:70110-61-1
- 5-Tricosyl-1,3-benzenediol
Catalog No.:BCN4262
CAS No.:70110-60-0
- 5-Heneicosylresorcinol
Catalog No.:BCN7630
CAS No.:70110-59-7
- TCN 237 dihydrochloride
Catalog No.:BCC6111
CAS No.:700878-19-9
- LY2157299
Catalog No.:BCC3709
CAS No.:700874-72-2
- LY2109761
Catalog No.:BCC3806
CAS No.:700874-71-1
- Acronycine
Catalog No.:BCC8114
CAS No.:7008-42-6
- Taranabant
Catalog No.:BCC1985
CAS No.:701977-09-5
- 3-Aminoadamantan-1-ol
Catalog No.:BCC8618
CAS No.:702-82-9
- 8alpha-Hydroxy-alpha-gurjunene
Catalog No.:BCN4265
CAS No.:70206-70-1
- (±)-Lauroylcarnitine chloride
Catalog No.:BCC6690
CAS No.:7023-03-2
- Physalin H
Catalog No.:BCN7917
CAS No.:70241-09-7
- BX-912
Catalog No.:BCC1250
CAS No.:702674-56-4
- BX795
Catalog No.:BCC3635
CAS No.:702675-74-9
- CP 775146
Catalog No.:BCC7881
CAS No.:702680-17-9
- Ivermectin
Catalog No.:BCC1251
CAS No.:70288-86-7
- Fluoroorotic Acid, Ultra Pure
Catalog No.:BCC1209
CAS No.:703-95-7
- Pertussis Toxin
Catalog No.:BCC7565
CAS No.:70323-44-3
- 3-Benzoylthiazolidine-2-thione
Catalog No.:BCC8624
CAS No.:70326-37-3
Inhibitor recognition specificity of MERS-CoV papain-like protease may differ from that of SARS-CoV.[Pubmed:25746232]
ACS Chem Biol. 2015 Jun 19;10(6):1456-65.
The Middle East Respiratory Syndrome coronavirus (MERS-CoV) papain-like protease (PLpro) blocking loop 2 (BL2) structure differs significantly from that of SARS-CoV PLpro, where it has been proven to play a crucial role in SARS-CoV PLpro inhibitor binding. Four SARS-CoV PLpro lead inhibitors were tested against MERS-CoV PLpro, none of which were effective against MERS-CoV PLpro. Structure and sequence alignments revealed that two residues, Y269 and Q270, responsible for inhibitor binding to SARS-CoV PLpro, were replaced by T274 and A275 in MERS-CoV PLpro, making critical binding interactions difficult to form for similar types of inhibitors. High-throughput screening (HTS) of 25000 compounds against both PLpro enzymes identified a small fragment-like noncovalent dual inhibitor. Mode of inhibition studies by enzyme kinetics and competition surface plasmon resonance (SPR) analyses suggested that this compound acts as a competitive inhibitor with an IC50 of 6 muM against MERS-CoV PLpro, indicating that it binds to the active site, whereas it acts as an allosteric inhibitor against SARS-CoV PLpro with an IC50 of 11 muM. These results raised the possibility that inhibitor recognition specificity of MERS-CoV PLpro may differ from that of SARS-CoV PLpro. In addition, inhibitory activity of this compound was selective for SARS-CoV and MERS-CoV PLpro enzymes over two human homologues, the ubiquitin C-terminal hydrolases 1 and 3 (hUCH-L1 and hUCH-L3).
Isolation and purification of a papain inhibitor from Egyptian genotypes of barley seeds and its in vitro and in vivo effects on the cowpea bruchid, Callosobruchus maculatus (F.).[Pubmed:25752426]
Pestic Biochem Physiol. 2015 Feb;118:26-32.
The cysteine inhibitors that are known as cystatin have been identified and characterized from several plant species. In the current study, 44 barley (Hordeum vulgare) genotypes including 3 varieties and 41 promising lines were screened for their potential as protease inhibitors. The barley genotypes showed low inhibitory activity against trypsin and chymotrypsin enzymes with a mean of 4.15 TIU/mg protein and 4.40 CIU/mg protein. The barley variety, Giza 123, showed strong Papain Inhibitory activity of 97.09 PIU/mg proteins and was subjected for further purification studies using ammonium sulfate fractionation and DEAE-Sephadex A-25 column. Barley purified proteins showed two bands on SDS-PAGE corresponding to a molecular mass of 12.4-54.8 kDa. The purified barley PI was found to be stable at a temperature below 80 degrees C and at a wide range of pH from 2 to 12. Barley PI was found to have higher potential inhibitory activity against papain enzyme compared to the standard Papain Inhibitor, E-64 with an IC50 value of 21.04 microg/ml and 25.62 microg/ml for barley PI and E-64, respectively. The kinetic analysis revealed a non-competitive type of inhibition with a Ki value of 1.95 x 10(-3 )microM. The antimetabolic effect of barley PI was evaluated against C. maculatus by incorporating the F30-60 protein of the purified inhibitor into the artificial diet using artificial seeds. Barley PI significantly prolonged the development of C. maculatus in proportion to PI concentration. Barley PI significantly increased the mortality of C. maculatus and caused a significant reduction in its fecundity. On the other hand, barley PI seemed to have non-significant effects on the adult longevity and the adult dry weight. The in vitro and in vivo results proved the efficiency of the Papain Inhibitory protein isolated from barley as a tool for managing the cowpea bruchid, C. maculatus.
An Unusual Member of the Papain Superfamily: Mapping the Catalytic Cleft of the Marasmius oreades agglutinin (MOA) with a Caspase Inhibitor.[Pubmed:26901797]
PLoS One. 2016 Feb 22;11(2):e0149407.
Papain-like cysteine proteases (PLCPs) constitute the largest group of thiol-based protein degrading enzymes and are characterized by a highly conserved fold. They are found in bacteria, viruses, plants and animals and involved in a number of physiological and pathological processes, parasitic infections and host defense, making them interesting targets for drug design. The Marasmius oreades agglutinin (MOA) is a blood group B-specific fungal chimerolectin with calcium-dependent proteolytic activity. The proteolytic domain of MOA presents a unique structural arrangement, yet mimicking the main structural elements in known PLCPs. Here we present the X-ray crystal structure of MOA in complex with Z-VAD-fmk, an irreversible caspase inhibitor known to cross-react with PLCPs. The structural data allow modeling of the substrate binding geometry and mapping of the fundamental enzyme-substrate interactions. The new information consolidates MOA as a new, yet strongly atypical member of the papain superfamily. The reported complex is the first published structure of a PLCP in complex with the well characterized caspase inhibitor Z-VAD-fmk.
Papain and its inhibitor E-64 reduce camelid semen viscosity without impairing sperm function and improve post-thaw motility rates.[Pubmed:27156102]
Reprod Fertil Dev. 2017 Jun;29(6):1107-1114.
In camelids, the development of assisted reproductive technologies is impaired by the viscous nature of the semen. The protease papain has shown promise in reducing viscosity, although its effect on sperm integrity is unknown. The present study determined the optimal papain concentration and exposure time to reduce seminal plasma viscosity and investigated the effect of papain and its inhibitor E-64 on sperm function and cryopreservation in alpacas. Papain (0.1mg mL(-1), 20min, 37 degrees C) eliminated alpaca semen viscosity while maintaining sperm motility, viability, acrosome integrity and DNA integrity. Furthermore E-64 (10 microM at 37 degrees C for 5min after 20min papain) inhibited the papain without impairing sperm function. Cryopreserved, papain-treated alpaca spermatozoa exhibited higher total motility rates after chilling and 0 and 1h after thawing compared with control (untreated) samples. Papain treatment, followed by inhibition of papain with E-64, is effective in reducing alpaca seminal plasma viscosity without impairing sperm integrity and improves post-thaw motility rates of cryopreserved alpaca spermatozoa. The use of the combination of papain and E-64 to eliminate the viscous component of camelid semen may aid the development of assisted reproductive technologies in camelids.


