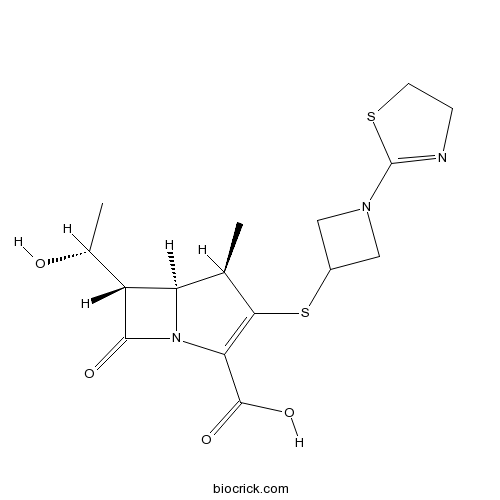
TebipenemCAS# 161715-21-5 |
- LKB1 (AAK1 dual inhibitor)
Catalog No.:BCC1705
CAS No.:1093222-27-5
- CX-6258
Catalog No.:BCC1504
CAS No.:1202916-90-2
- CX-6258 hydrochloride hydrate
Catalog No.:BCC1505
CAS No.:1353858-99-7
- PIM-1 Inhibitor 2
Catalog No.:BCC2446
CAS No.:477845-12-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 161715-21-5 | SDF | Download SDF |
| PubChem ID | 9800194 | Appearance | Powder |
| Formula | C16H21N3O4S2 | M.Wt | 383.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | LJC 11036 | ||
| Solubility | DMSO : 33.33 mg/mL (86.91 mM; Need ultrasonic) H2O : 7.14 mg/mL (18.62 mM; Need ultrasonic) | ||
| Chemical Name | (4R,5S,6S)-3-[1-(4,5-dihydro-1,3-thiazol-2-yl)azetidin-3-yl]sulfanyl-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid | ||
| SMILES | CC1C2C(C(=O)N2C(=C1SC3CN(C3)C4=NCCS4)C(=O)O)C(C)O | ||
| Standard InChIKey | GXXLUDOKHXEFBQ-YJFSRANCSA-N | ||
| Standard InChI | InChI=1S/C16H21N3O4S2/c1-7-11-10(8(2)20)14(21)19(11)12(15(22)23)13(7)25-9-5-18(6-9)16-17-3-4-24-16/h7-11,20H,3-6H2,1-2H3,(H,22,23)/t7-,8-,10-,11-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Tebipenem Dilution Calculator

Tebipenem Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6076 mL | 13.0381 mL | 26.0763 mL | 52.1526 mL | 65.1907 mL |
| 5 mM | 0.5215 mL | 2.6076 mL | 5.2153 mL | 10.4305 mL | 13.0381 mL |
| 10 mM | 0.2608 mL | 1.3038 mL | 2.6076 mL | 5.2153 mL | 6.5191 mL |
| 50 mM | 0.0522 mL | 0.2608 mL | 0.5215 mL | 1.0431 mL | 1.3038 mL |
| 100 mM | 0.0261 mL | 0.1304 mL | 0.2608 mL | 0.5215 mL | 0.6519 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tebipenem is an orally available carbapenem antibiotic, shows broad-spectrum activity against Gram-positive and -negative bacteria, except for Pseudomonas aeruginosa.
In Vitro:Tebipenem exhibits slow tight-binding inhibition at low micromolar concentrations versus the chromogenic substrate nitrocefin, and apparent Km and kcat values of 0.8 μM and 0.03 min-1, respectively[1]. Tebipenem shows potent activity against B. pseudomallei, with MIC50 and MIC90 values of both 2 mg/L[2]. Tebipenem shows good activity against S. pneumoniae, with the MIC range of ≤0.25 μg/mL in all of the S. pneumoniae isolates[3].
References:
[1]. Hazra S, et al. Tebipenem, a new carbapenem antibiotic, is a slow substrate that inhibits the β-lactamase from Mycobacterium tuberculosis. Biochemistry. 2014 Jun 10;53(22):3671-8.
[2]. Seenama C, et al. In vitro activity of tebipenem against Burkholderia pseudomallei. Int J Antimicrob Agents. 2013 Oct;42(4):375.
[3]. Li H, et al. In vitro antibacterial activities of two novel oral antibiotics, tebipenem and cefditoren, and other comparators against community-acquired respiratory tract infection-associated bacterial pathogens: A multicentre study in China. Int J Antimicrob Agents. 2014 Jan;43(1):92-3.
- Z-Ile-Leu-aldehyde
Catalog No.:BCC5591
CAS No.:161710-10-7
- Vincamine
Catalog No.:BCN2606
CAS No.:1617-90-9
- Lupenone
Catalog No.:BCN1717
CAS No.:1617-70-5
- Lupeol acetate
Catalog No.:BCN6893
CAS No.:1617-68-1
- Amentoflavone
Catalog No.:BCN6283
CAS No.:1617-53-4
- 2,3,8-Tri-O-methylellagic acid
Catalog No.:BCN1716
CAS No.:1617-49-8
- Caesalpine B
Catalog No.:BCN7377
CAS No.:1616757-60-8
- Caesalpine A
Catalog No.:BCN7376
CAS No.:1616757-59-5
- Dodovislactone B
Catalog No.:BCN7398
CAS No.:1616683-55-6
- Dodovislactone A
Catalog No.:BCN7399
CAS No.:1616683-54-5
- Dodovisone D
Catalog No.:BCN6871
CAS No.:1616683-53-4
- Dodovisone C
Catalog No.:BCN6872
CAS No.:1616683-52-3
- Tebipenempivoxil
Catalog No.:BCC3861
CAS No.:161715-24-8
- Rasagiline mesylate
Catalog No.:BCN2166
CAS No.:161735-79-1
- GLP-1 (9-36) amide
Catalog No.:BCC6001
CAS No.:161748-29-4
- 12-Oxocalanolide A
Catalog No.:BCN4699
CAS No.:161753-49-7
- Palmatrubine
Catalog No.:BCN2647
CAS No.:16176-68-4
- Esomeprazole Sodium
Catalog No.:BCC4376
CAS No.:161796-78-7
- Ethyl 2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylate
Catalog No.:BCC8969
CAS No.:161797-99-5
- Ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate
Catalog No.:BCC8968
CAS No.:161798-01-2
- Ethyl 2-(3-cyano-4-hydroxyphenyl)-4-methyl-1,3-thiazole-5-carboxylate
Catalog No.:BCC8966
CAS No.:161798-02-3
- Benzamil
Catalog No.:BCC7674
CAS No.:161804-20-2
- Amprenavir (agenerase)
Catalog No.:BCC3619
CAS No.:161814-49-9
- Talampanel(LY300164)
Catalog No.:BCC6378
CAS No.:161832-65-1
Antibacterial Properties of Tebipenem Pivoxil Tablet, a New Oral Carbapenem Preparation against a Variety of Pathogenic Bacteria in Vitro and in Vivo.[Pubmed:26751436]
Molecules. 2016 Jan 6;21(1):62.
AIMS: To systemically investigate the in vitro and in vivo antibacterial properties of Tebipenem pivoxil tablet. In addition, acute toxicity of this preparation was also studied. METHODS: In vitro, minimum inhibitory concentration (MIC) or minimal inhibitory concentration (MBC) were determined by using the serial 2-fold broth or agar dilution methods. Further, cumulative MIC inhibition curves were then made to assess the antibacterial effects of the drug at various concentrations. In vivo, minimum lethal dose (MLD) in combination with maximum tolerance dose (MTD) was used to measure the acute toxicity of the Tebipenem pivoxil tablet in mice. After that, sepsis mouse models challenged with Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Klebsiella pneumoniae, respectively, were established to evaluate the anti-infective effect of this preparation. RESULTS: The MIC90 values of Tebipenem pivoxil against Gram-positive bacteria such as methicillin-sensitive Staphylococcus aureus (MSSA), methicillin-resistant Staphylococcus aureus (MRSA), methicillin-sensitive Staphylococcus epidermidis (MSSE), methicillin-resistant Staphylococcus epidermidis (MRSE), Pyogenic streptococcus, and Enterococcus faecalis were Tebipenem pivoxil against Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Haemophilus influenzae, Pseudomonas aeruginosa, and Acinetobacter baumannii were 1, 0.5, Tebipenem pivoxil against Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae were 0.016-2, 0.063-32, 0.031-32 mug/mL, respectively. The acute toxicity study showed that the MLD of the Tebipenem pivoxil tablet was 4.00 g/kg and the MTD was 3.40 g/kg in mice. In all the sepsis mouse models, the simultaneous administration of the Tebipenem pivoxil tablets significantly reduced mortality of the sepsis-model mice as compared with the control. Furthermore, the survival rate in the Tebipenem pivoxil tablet group was remarkably higher than that in the meropenem group in all the sepsis mouse models tested. In the sepsis model challenged with Staphylococcus aureus ATCC29213, Escherichia coli ATCC25922, Pseudomonas aeruginosa ATCC27853, and Pseudomonas aeruginosa clinical strain, respectively, Tebipenem pivoxil tablet (100 mg/kg) displayed a better protective effect than Tebipenem pivoxil granules (100 mg/kg). CONCLUSIONS: In summary, Tebipenem pivoxil displays an excellent antibacterial activity against a variety of pathogenic bacteria in vitro. Importantly, Tebipenem pivoxil tablet significantly protects the sepsis mice challenged with various pathogenic bacteria, which may provide a potential approach to treating bacterial sepsis in clinic.
Pediatric community-acquired pneumonia treated with a three-day course of tebipenem pivoxil.[Pubmed:28238680]
J Infect Chemother. 2017 May;23(5):307-311.
We evaluated the efficacy and safety of a 3-day treatment regimen of Tebipenem pivoxil for pediatric community-acquired pneumonia. Tebipenem pivoxil was administered to 49 patients, and its effectiveness was evaluated in 36 patients 2-4 days after initiation of treatment. Thirty-two patients were cured 7-15 days after initiation of treatment. Body temperature was significantly lower on the day following initial administration (median 38.8 to 37.0 degrees C, n = 33). Leukocyte counts and C-reactive protein levels were significantly reduced by Day 2-4 of treatment (median 16,100 to 7800 white blood cells/muL, and 5.6 to 1.5 mg/dL, respectively; n = 28). Six of the 49 patients had mild diarrhea. Thus, we concluded that 3-day treatment with Tebipenem pivoxil was safe and efficacious for treating pediatric community-acquired pneumonia.
Solid-state stability studies of crystal form of tebipenem.[Pubmed:26043654]
Drug Dev Ind Pharm. 2016;42(2):238-44.
The aim of this study was to determine the kinetic and thermodynamic parameters of Tebipenem degradation in the solid state. The process was analyzed based on the results obtained by a high performance liquid chromatography (HPLC) method using ultraviolet diode-array detector (DAD)/electrospray ionization tandem mass spectrometry (Q-TOF-MS/MS), Fourier transform infrared spectroscopy (FT-IR) and Raman spectroscopic (RS) studies. In dry air, the degradation of Tebipenem was a first-order reaction depending on the substrate concentration while at an increased relative air humidity Tebipenem was degraded according to the kinetic model of autocatalysis. The thermodynamic parameters: energy of activation (Ea), enthalpy (DeltaH( not equala)) and entropy (DeltaS( not equala)) of Tebipenem degradation were calculated. Following a spectroscopic analysis of degraded samples of Tebipenem, a cleavage of the beta-lactam bond was proposed as the main degradation pathway, next confirmation using HPLC-Q-TOF-MS/MS method.
[Evaluation of safety and efficacy of tebipenem pivoxil granules for pediatric in pneumonia, otitis media and sinusitis].[Pubmed:27290830]
Jpn J Antibiot. 2016 Feb;69(1):53-76.
We conducted a postmarketing surveillance of Tebipenem pivoxil granules (Orapenem(R) fine granules 10% for pediatric), an oral carbapenem antibacterial agent, between April 2010 and March 2013 to evaluate the safety and efficacy in patients with pneumonia or otitis media, or sinusitis Of 3,547 patients enrolled, 3,540 from whom survey forms were collected were analyzed. Of these 3,540 patients, there were a total of 3,331 patients included in the safety analysis, 2,844 in the efficacy analysis, 2,769 in the clinical efficacy analysis, and 461 in the bacteriological efficacy analysis. The incidence of adverse drug reactions (ADRs) was 9.97% (332/3,331 patients), and the major ADRs were gastrointestinal disorders including diarrhoea in 317 patients (9.52%). Diarrhoea was reported in 313 patients (316 events), which were not clinically significant and 94.9% (297/313 patients) were recovery and/or remission. The overall clinical efficacy rate was 94.0% (2,604/2,769 patients). The clinical efficacy rate by the type of infection was 95.6% (415/434 patients) for pneumonia, 93.7% (1,389/1,482 patients) for otitis media and 93.6% (659/704 patients) for sinusitis. The eradication rate of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella (Branhamella) catarrhalis which are major causative organisms in pediatric infection of pneumonia, otitis media and sinusitis were 94.4% (134/142 strains), 92.2% (130/141 strains) and 97.8% (45/46 strains), respectively. The compliance was good in 83.1% of the patients (2,767/3,331 patients). Overall, Orapenem(R) fine granules 10% for pediatric showed good safety, efficacy, and compliance. These results indicate that Orapenem(R) fine granules 10% for pediatric is a useful agent in pediatrics with pneumonia or otitis media, or sinusitis.


