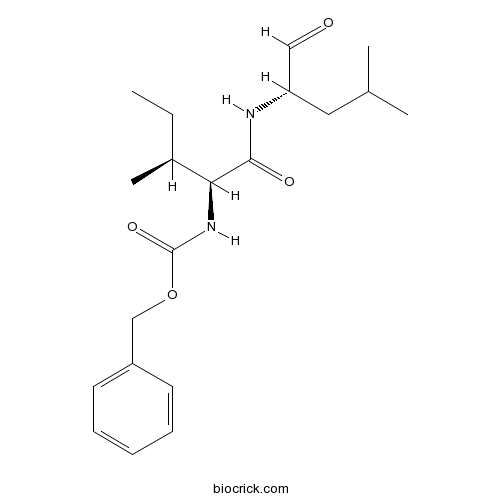
Z-Ile-Leu-aldehydeCAS# 161710-10-7 |
- Q-VD-OPh hydrate
Catalog No.:BCC1125
CAS No.:1135695-98-5
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Caspase-3/7 Inhibitor I
Catalog No.:BCC1140
CAS No.:220509-74-0
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 161710-10-7 | SDF | Download SDF |
| PubChem ID | 44366908 | Appearance | Powder |
| Formula | C20H30N2O4 | M.Wt | 362.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Z-IL-CHO; GSI-XII; γ-Secretase inhibitor XII | ||
| Solubility | >10.8mg/mL in DMSO | ||
| Chemical Name | benzyl N-[(2S,3S)-3-methyl-1-[[(2S)-4-methyl-1-oxopentan-2-yl]amino]-1-oxopentan-2-yl]carbamate | ||
| SMILES | CCC(C)C(C(=O)NC(CC(C)C)C=O)NC(=O)OCC1=CC=CC=C1 | ||
| Standard InChIKey | WJQLUFQGNVGLKR-SZMVWBNQSA-N | ||
| Standard InChI | InChI=1S/C20H30N2O4/c1-5-15(4)18(19(24)21-17(12-23)11-14(2)3)22-20(25)26-13-16-9-7-6-8-10-16/h6-10,12,14-15,17-18H,5,11,13H2,1-4H3,(H,21,24)(H,22,25)/t15-,17-,18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Z-Ile-Leu-aldehyde Dilution Calculator

Z-Ile-Leu-aldehyde Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7589 mL | 13.7946 mL | 27.5893 mL | 55.1785 mL | 68.9731 mL |
| 5 mM | 0.5518 mL | 2.7589 mL | 5.5179 mL | 11.0357 mL | 13.7946 mL |
| 10 mM | 0.2759 mL | 1.3795 mL | 2.7589 mL | 5.5179 mL | 6.8973 mL |
| 50 mM | 0.0552 mL | 0.2759 mL | 0.5518 mL | 1.1036 mL | 1.3795 mL |
| 100 mM | 0.0276 mL | 0.1379 mL | 0.2759 mL | 0.5518 mL | 0.6897 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Z-Ile-Leu-aldehyde(Z-IL-CHO; GSI-XII) is a potent gamma-Secretase inhibitor; Notch signaling inhibitor. IC50 value: Target: gamma-Secretase inhibitor in vitro: GSI-XII induces apoptosis of murine MOPC315.BM myeloma cells with high Notch activity. GSI XII impairs murine osteoclast differentiation of receptor activator of NF-κB ligand (RANKL)-stimulated RAW264.7 cells in vitro [1]. Notch-signaling inhibition in HRS cells by the γ-secretase inhibitor (GSI) XII results in decreased alternative p52/RelB NF-κB signaling, interfering with processing of the NF-κB2 gene product p100 into its active form p52 [2]. GSI treatment induced morphologic erythroid differentiation and promoted hemoglobin production. GSI treatment suppressed short-term growth and colony formation, while treatment with GSI-XXI promoted the growth of AA cells [3]. in vivo: In the murine MOPC315.BM myeloma model GSI XII has potent anti-MM activity and reduces osteolytic lesions as evidenced by diminished myeloma-specific monoclonal immunoglobulin (Ig)-A serum levels and quantitative assessment of bone structure changes via high-resolution microcomputed tomography scans [1].
References:
[1]. Schwarzer R, et al. Notch pathway inhibition controls myeloma bone disease in the murine MOPC315.BM model. Blood Cancer J. 2014 Jun 13;4:e217.
[2]. Schwarzer R, et al. Notch is an essential upstream regulator of NF-κB and is relevant for survival of Hodgkin and Reed-Sternberg cells. Leukemia. 2012 Apr;26(4):806-13.
[3]. Okuhashi Y, et al. Gamma-secretase inhibitors induce erythroid differentiation in erythroid leukemia cell lines. Anticancer Res. 2010 Oct;30(10):4071-4.
- Vincamine
Catalog No.:BCN2606
CAS No.:1617-90-9
- Lupenone
Catalog No.:BCN1717
CAS No.:1617-70-5
- Lupeol acetate
Catalog No.:BCN6893
CAS No.:1617-68-1
- Amentoflavone
Catalog No.:BCN6283
CAS No.:1617-53-4
- 2,3,8-Tri-O-methylellagic acid
Catalog No.:BCN1716
CAS No.:1617-49-8
- Caesalpine B
Catalog No.:BCN7377
CAS No.:1616757-60-8
- Caesalpine A
Catalog No.:BCN7376
CAS No.:1616757-59-5
- Dodovislactone B
Catalog No.:BCN7398
CAS No.:1616683-55-6
- Dodovislactone A
Catalog No.:BCN7399
CAS No.:1616683-54-5
- Dodovisone D
Catalog No.:BCN6871
CAS No.:1616683-53-4
- Dodovisone C
Catalog No.:BCN6872
CAS No.:1616683-52-3
- Dodovisone B
Catalog No.:BCN6867
CAS No.:1616683-51-2
- Tebipenem
Catalog No.:BCC5550
CAS No.:161715-21-5
- Tebipenempivoxil
Catalog No.:BCC3861
CAS No.:161715-24-8
- Rasagiline mesylate
Catalog No.:BCN2166
CAS No.:161735-79-1
- GLP-1 (9-36) amide
Catalog No.:BCC6001
CAS No.:161748-29-4
- 12-Oxocalanolide A
Catalog No.:BCN4699
CAS No.:161753-49-7
- Palmatrubine
Catalog No.:BCN2647
CAS No.:16176-68-4
- Esomeprazole Sodium
Catalog No.:BCC4376
CAS No.:161796-78-7
- Ethyl 2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylate
Catalog No.:BCC8969
CAS No.:161797-99-5
- Ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate
Catalog No.:BCC8968
CAS No.:161798-01-2
- Ethyl 2-(3-cyano-4-hydroxyphenyl)-4-methyl-1,3-thiazole-5-carboxylate
Catalog No.:BCC8966
CAS No.:161798-02-3
- Benzamil
Catalog No.:BCC7674
CAS No.:161804-20-2
- Amprenavir (agenerase)
Catalog No.:BCC3619
CAS No.:161814-49-9


