Caspase-3/7 Inhibitor ICaspase-3/7 inhibitor CAS# 220509-74-0 |
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Z-DEVD-FMK
Catalog No.:BCC1137
CAS No.:210344-95-9
- VX-765
Catalog No.:BCC3648
CAS No.:273404-37-8
- Apoptosis Inhibitor
Catalog No.:BCC1143
CAS No.:54135-60-3
- Ivachtin
Catalog No.:BCC2357
CAS No.:745046-84-8
- Apoptosis Activator 2
Catalog No.:BCC2099
CAS No.:79183-19-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 220509-74-0 | SDF | Download SDF |
| PubChem ID | 9840223 | Appearance | Powder |
| Formula | C14H16N2O5S | M.Wt | 324.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >16.2mg/mL in DMSO | ||
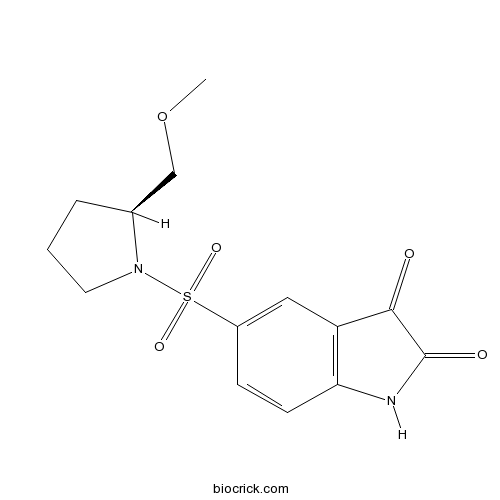
| Chemical Name | 5-[(2S)-2-(methoxymethyl)pyrrolidin-1-yl]sulfonyl-1H-indole-2,3-dione | ||
| SMILES | COCC1CCCN1S(=O)(=O)C2=CC3=C(C=C2)NC(=O)C3=O | ||
| Standard InChIKey | SLQMNVJNDYLJSF-VIFPVBQESA-N | ||
| Standard InChI | InChI=1S/C14H16N2O5S/c1-21-8-9-3-2-6-16(9)22(19,20)10-4-5-12-11(7-10)13(17)14(18)15-12/h4-5,7,9H,2-3,6,8H2,1H3,(H,15,17,18)/t9-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A potent, cell-permeable, and specific, reversible inhibitor of caspase-3 (Ki = 60 nM) and caspase-7 (Ki = 170 nM). | |||||
| Targets | Caspase-3 | Caspase-7 | ||||
| IC50 | 60nM | 170nM | ||||
| Cell experiment: [1] | |
| Cell lines | Human Jurkat T cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 50 μM |
| Applications | Cells were treated with camptothecin to induce cell death, and the ability of the compound to inhibit cell death was assessed by FACS analysis. A good correlation exists between relative cell-based activities of the compound with its in vitro isolated caspase 3 or 7 inhibition activites. The compound exhibited 54% inhibition of apoptosis at 50 μM and 22% at 10 μM. |
| Animal experiment: | |
| Animal models |
|
| Dosage form |
|
| Application |
|
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Lee D, Long S A, Murray J H, et al. Potent and selective nonpeptide inhibitors of caspases 3 and 7. Journal of medicinal chemistry, 2001, 44(12): 2015-2026. | |

Caspase-3/7 Inhibitor I Dilution Calculator

Caspase-3/7 Inhibitor I Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0826 mL | 15.4131 mL | 30.8261 mL | 61.6523 mL | 77.0654 mL |
| 5 mM | 0.6165 mL | 3.0826 mL | 6.1652 mL | 12.3305 mL | 15.4131 mL |
| 10 mM | 0.3083 mL | 1.5413 mL | 3.0826 mL | 6.1652 mL | 7.7065 mL |
| 50 mM | 0.0617 mL | 0.3083 mL | 0.6165 mL | 1.233 mL | 1.5413 mL |
| 100 mM | 0.0308 mL | 0.1541 mL | 0.3083 mL | 0.6165 mL | 0.7707 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
TIMP-3 is involved in the regulation of inflammation where, in the absence of TIMP3, macrophages are more likely to be differentiated into proinflammatory (M1) cells.
Abstract
The effects of inhibition of XIAP, Survivin or both on cell proliferation and chemosensitivity of Panc-1 cells have been investigated and compared.
Abstract
The effect of AZD1152 alone or in combination with cisplatin has been investigated in the treatment of cisplatin-resistant ovarian carcinoma.
Abstract
The regulation of XIAP gene by lentivirus-mediated shRNA has been investigated for its effect in the treatment of pancreatic cancer.
Abstract
Z-DEVD, a selective caspase 3/7 inhibitor, didn’t affect the sensitivity of three mesothelioma-derived cell lines to cisplatin.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Caspase-3/7 inbibitor I is a potent, reversible, isatin sulfonamide-based inhibitor of caspase-3 (KI(app) = 60 nM) and caspase-7 (KI(app) = 170 nM). Is a weaker inhibitor of caspase-9 (Ki(app) = 3.1 mM). It Has only a trivial effect (Ki(app) >25 mM) on the activities of caspase-1, caspase-2, caspase-4, caspase-6, and caspase-8. It has been shown to inhibit apoptosis in camptothecin treated Jurkat cells (IC50 ~50 µM). Also it has been reported to inhibit apoptosis in chondrocytes (44% inhibition at 10 µM and 98% inhibition at 50 µM). Selectivity for caspases-3 and 7 involves unique hydrophobic residues in the S2 pocket surrounding the catalytic cysteine residue. [1] [2] In some systems inhibition of caspases-3 and -7 can prevent apoptosis and may therefore have important therapeutic implications. [3]
A potent, cell-permeable, and specific, reversible inhibitor of caspase-3 (Ki = 60 nM) and caspase-7 (Ki = 170 nM).
References:
1. Lee, D., et al. 2001. J. Med. Chem. 44, 2015.
2. Lee, D., et al. 2000. J. Biol. Chem. 275, 16007.
3. Clements, K. M., Burton‐Wurster, N., Nuttall, M. E., & Lust, G. (2005). Caspase‐3/7 inhibition alters cell morphology in mitomycin‐c treated chondrocytes. Journal of cellular physiology, 205(1), 133-140.
- 1-Methyl-2-pentyl-4(1H)-quinolinone
Catalog No.:BCN4943
CAS No.:22048-98-2
- Leptomerine
Catalog No.:BCN1486
CAS No.:22048-97-1
- Kaempferol 5-methyl ether
Catalog No.:BCN3426
CAS No.:22044-80-0
- Polycephalin C
Catalog No.:BCN1852
CAS No.:220422-37-7
- ShK-Dap22
Catalog No.:BCC5990
CAS No.:220384-25-8
- 5-Epicanadensene
Catalog No.:BCN7349
CAS No.:220384-17-8
- DH 97
Catalog No.:BCC6973
CAS No.:220339-00-4
- TRO 19622
Catalog No.:BCC5288
CAS No.:22033-87-0
- 3,11,12-Trihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1487
CAS No.:220328-04-1
- 5-[2-[Tert-butyl(dimethyl)silyl]oxyethyl]-2,2-dimethyl-3a,6a-dihydrofuro[2,3-d][1,3]dioxol-6-one
Catalog No.:BCC8592
CAS No.:220328-03-0
- Cinnamamide
Catalog No.:BCN4942
CAS No.:22031-64-7
- Triptohairic acid
Catalog No.:BCN8060
CAS No.:220209-71-2
- Diphyllin
Catalog No.:BCN8066
CAS No.:22055-22-7
- GB 1a
Catalog No.:BCN7237
CAS No.:220611-41-6
- Tigecycline
Catalog No.:BCC2499
CAS No.:220620-09-7
- RJR-2403 oxalate
Catalog No.:BCC1902
CAS No.:220662-95-3
- Antisauvagine-30
Catalog No.:BCC5868
CAS No.:220673-95-0
- Oxonic acid potassium salt
Catalog No.:BCC4165
CAS No.:2207-75-2
- Ketoprofen
Catalog No.:BCC4430
CAS No.:22071-15-4
- Curlignan
Catalog No.:BCN3977
CAS No.:220736-54-9
- Aquifoliunine E-III
Catalog No.:BCN3096
CAS No.:220751-20-2
- Aminopurvalanol A
Catalog No.:BCC7249
CAS No.:220792-57-4
- NG,NG-Dimethylarginine dihydrochloride
Catalog No.:BCN1485
CAS No.:220805-22-1
- 7-Methoxy-8-Hydroxy-4-Methylcoumarin
Catalog No.:BCC8291
CAS No.:22084-94-2
Synthesis of 7-halogenated isatin sulfonamides: nonradioactive counterparts of caspase-3/-7 inhibitor-based potential radiopharmaceuticals for molecular imaging of apoptosis.[Pubmed:25358116]
J Med Chem. 2014 Nov 26;57(22):9383-95.
N-Alkylated (S)-7-halogen-5-[1-(2-methoxymethylpyrrolidinyl)sulfonyl]isatins were developed as a new group of nonradioactive reference compounds for future radiotracers. Inhibitor potency studies of these compounds suggest that the binding pockets readily accommodate both the 7-halogen substituents and aliphatic side chains (methyl to n-butyl) as well as some omega-fluorinated analogues (3-fluoropropyl and 4-fluorobutyl) at the isatin nitrogen. Indeed, compared to the halogen free parent compounds, some 7-halogenated derivatives exhibited slightly improved inhibitory potencies with IC50 values up to 2.6 nM (caspase-3) and 3.3 nM (caspase-7), respectively. Moreover, the 7-position of isatin, a potential cytochrome P450 hydroxylation site, was substituted by I, Br, Cl, and F to potentially enhance the metabolic stability of isatin sulfonamides. As an example, the radiotracer [(18)F]39 that was produced by (19)F/(18)F isotope exchange was shown to be stable in human blood serum after incubation at 37 degrees C for at least 90 min.
A novel nonpeptidic caspase-3/7 inhibitor, (S)-(+)-5-[1-(2-methoxymethylpyrrolidinyl)sulfonyl]isatin reduces myocardial ischemic injury.[Pubmed:12450570]
Eur J Pharmacol. 2002 Dec 5;456(1-3):59-68.
The efficacy of a novel, nonpeptidic, caspase 3/7-selective inhibitor, (S)-(+)-5-[1-(2-methoxymethylpyrrolidinyl)sulfonyl]isatin (MMPSI) for reducing ischemic injury in isolated rabbit hearts or cardiomyocytes was evaluated. MMPSI (0.1-10 microM) evoked a concentration-dependent reduction in infarct size (up to 56% vs. control; IC(50)=0.2 microM). Furthermore, apoptosis (DNA laddering, soluble nucleosomes) was reduced in the ischemic area-at-risk. MMPSI inhibited recombinant human caspase-3 with an IC(50)=1.7 microM. Apoptosis in H9c2 cells after 16-h simulated ischemia and 2-h simulated reperfusion was significantly reduced by MMPSI in a concentration-dependent manner (IC(50)=0.5 microM); similar effects were observed in isolated adult rabbit cardiomyocytes (IC(50)=1.5 microM). These data support an important role for caspase-3/7 in mediating myocardial ischemic injury. Furthermore, these data indicate that cardioprotection via caspase-3/7 inhibition is attainable via a small molecule (nonpeptidic) inhibitor, a necessary step in making this approach therapeutically viable.
Combination of the histone deacetylase inhibitor depsipeptide and 5-fluorouracil upregulates major histocompatibility complex class II and p21 genes and activates caspase-3/7 in human colon cancer HCT-116 cells.[Pubmed:27509880]
Oncol Rep. 2016 Oct;36(4):1875-85.
Epigenetic anticancer drugs such as histone deacetylase (HDAC) inhibitors have been combined with existing anticancer drugs for synergistic or additive effects. In the present study, we found that a very low concentration of depsipeptide, an HDAC inhibitor, potentiated the antitumor activity of 5-fluorouracil (5-FU) in a human colon cancer cell model using HCT-116, HT29, and SW48 cells via the inhibition of colony formation ability or cellular viability. Exposure to a combination of 5-FU (1.75 microM) and 1 nM depsipeptide for 24 and 48 h resulted in a 3- to 4-fold increase in activated caspase-3/7, while 5-FU alone failed to activate caspase-3/7. Microarray and subsequent gene ontology analyses revealed that compared to 5-FU or depsipeptide alone, the combination treatment of 5-FU and depsipeptide upregulated genes related to cell death and the apoptotic process consistent with the inhibition of colony formation and caspase-3/7 activation. These analyses indicated marked upregulation of antigen processing and presentation of peptide or polysaccharide antigen via major histocompatibility complex (MHC) class (GO:0002504) and MHC protein complex (GO:0042611). Compared with vehicle controls, the cells treated with the combination of 5-FU and depsipeptide showed marked induction (3- to 8.5-fold) of expression of MHC class II genes, but not of MHC class I genes. Furthermore, our global analysis of gene expression, which was focused on genes involved in the molecular regulation of MHC class II genes, showed enhancement of pro-apoptotic PCAF and CIITA after the combination of 5-FU and depsipeptide. These results may indicate a closer relationship between elevation of MHC class II expression and cellular apoptosis induced by the combination of depsipeptide and 5-FU. To the best of our knowledge, this is the first study to report that the combination of 5-FU and depsipeptide induces human colon cancer cell apoptosis in a concerted manner with the induction of MHC class II gene expression.


