Oxonic acid potassium saltUricase inhibitor CAS# 2207-75-2 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2207-75-2 | SDF | Download SDF |
| PubChem ID | 2723920 | Appearance | Powder |
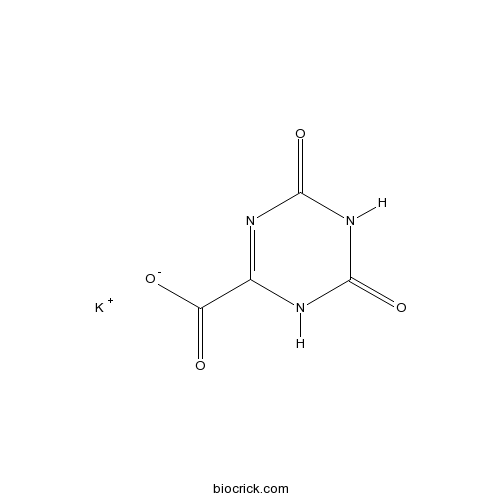
| Formula | C4H2KN3O4 | M.Wt | 195.17 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Potassium azaorotate; Potassium otastat; Potassium oxonate | ||
| Solubility | H2O : 9.09 mg/mL (46.57 mM; Need ultrasonic) DMSO : < 1 mg/mL (insoluble or slightly soluble) | ||
| Chemical Name | potassium;4,6-dioxo-1H-1,3,5-triazine-2-carboxylate | ||
| SMILES | C1(=NC(=O)NC(=O)N1)C(=O)[O-].[K+] | ||
| Standard InChIKey | IAPCTXZQXAVYNG-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C4H3N3O4.K/c8-2(9)1-5-3(10)7-4(11)6-1;/h(H,8,9)(H2,5,6,7,10,11);/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Oxonic acid potassium salt is an inhibitor of uricase, oxonic inhibits the phosphorylation of 5-FU to 5-fluorouridine-5'-monophosphate catalyzed by pyrimidine phosphoribosyl-transferase in a different manner from allopurinol in cell-free extracts and intact cells in vitro.
IC50 value:
Target:
On p.o. administration of 5-FU (2 mg/kg) and a potent inhibitor of 5-FU degradation to Yoshida sarcoma-bearing rats, oxonic acid (10 mg/kg) was found to inhibit the formation of 5-fluorouridine-5'-monophosphate from 5-FU and its subsequent incorporation into the RNA fractions of small and large intestine but not of tumor and bone marrow tissues [1]. Oxonic acid diet increased plasma uric acid by 80-90 micromol/l, while blood pressure was elevated only in hyperuricemic 5/6 nephrectomy rats (18 mmHg) [2]. References: | |||||

Oxonic acid potassium salt Dilution Calculator

Oxonic acid potassium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.1237 mL | 25.6187 mL | 51.2374 mL | 102.4748 mL | 128.0935 mL |
| 5 mM | 1.0247 mL | 5.1237 mL | 10.2475 mL | 20.495 mL | 25.6187 mL |
| 10 mM | 0.5124 mL | 2.5619 mL | 5.1237 mL | 10.2475 mL | 12.8093 mL |
| 50 mM | 0.1025 mL | 0.5124 mL | 1.0247 mL | 2.0495 mL | 2.5619 mL |
| 100 mM | 0.0512 mL | 0.2562 mL | 0.5124 mL | 1.0247 mL | 1.2809 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Oxonic acid potassium salt is an inhibitor of uricase, oxonic inhibits the phosphorylation of 5-FU to 5-fluorouridine-5'-monophosphate catalyzed by pyrimidine phosphoribosyl-transferase in a different manner from allopurinol in cell-free extracts and inta
- Antisauvagine-30
Catalog No.:BCC5868
CAS No.:220673-95-0
- RJR-2403 oxalate
Catalog No.:BCC1902
CAS No.:220662-95-3
- Tigecycline
Catalog No.:BCC2499
CAS No.:220620-09-7
- GB 1a
Catalog No.:BCN7237
CAS No.:220611-41-6
- Diphyllin
Catalog No.:BCN8066
CAS No.:22055-22-7
- Caspase-3/7 Inhibitor I
Catalog No.:BCC1140
CAS No.:220509-74-0
- 1-Methyl-2-pentyl-4(1H)-quinolinone
Catalog No.:BCN4943
CAS No.:22048-98-2
- Leptomerine
Catalog No.:BCN1486
CAS No.:22048-97-1
- Kaempferol 5-methyl ether
Catalog No.:BCN3426
CAS No.:22044-80-0
- Polycephalin C
Catalog No.:BCN1852
CAS No.:220422-37-7
- ShK-Dap22
Catalog No.:BCC5990
CAS No.:220384-25-8
- 5-Epicanadensene
Catalog No.:BCN7349
CAS No.:220384-17-8
- Ketoprofen
Catalog No.:BCC4430
CAS No.:22071-15-4
- Curlignan
Catalog No.:BCN3977
CAS No.:220736-54-9
- Aquifoliunine E-III
Catalog No.:BCN3096
CAS No.:220751-20-2
- Aminopurvalanol A
Catalog No.:BCC7249
CAS No.:220792-57-4
- NG,NG-Dimethylarginine dihydrochloride
Catalog No.:BCN1485
CAS No.:220805-22-1
- 7-Methoxy-8-Hydroxy-4-Methylcoumarin
Catalog No.:BCC8291
CAS No.:22084-94-2
- Thunalbene
Catalog No.:BCN3688
CAS No.:220862-05-5
- 2,3-O-Isopropylidenyl euscaphic acid
Catalog No.:BCN4944
CAS No.:220880-90-0
- GW5074
Catalog No.:BCC4391
CAS No.:220904-83-6
- 13-O-Cinnamoylbaccatin III
Catalog No.:BCN7344
CAS No.:220932-65-0
- 13-O-Deacetyltaxumairol Z
Catalog No.:BCN4945
CAS No.:220935-39-7
- Antalarmin hydrochloride
Catalog No.:BCC7480
CAS No.:220953-69-5
Study on the correlation between constituents detected in serum from Rhizoma Smilacis Glabrae and the reduction of uric acid levels in hyperuricemia.[Pubmed:24140588]
J Ethnopharmacol. 2013 Nov 25;150(2):747-54.
ETHNOPHARMACOLOGICAL RELEVANCE: Rhizoma Smilacis Glabrae (RSG) has been used in the clinical treatment of gout and hyperuricemia in China for thousands of years. Modern pharmacological studies have shown that RSG exhibits hypouricemic effects because of its significant inhibitory effect on the activity of xanthine oxidase. MATERIALS AND METHODS: The Rhizoma Smilacis Glabrae extract (RSGE) at 1 mL/100g oral administration was demonstrated to possess in vivo potent hypouricemic effects in hyperuricemic rats pretreated with Oxonic acid potassium salt (200 mg/kg, 2 mL/kg). UPLC-MS was used to identify the constituents absorbed in the serum. In addition, a bivariate correlation analysis between the changes in the relative contents of the constituents from RSGE detected by HPLC and the serum uric acid levels in hyperuricemic rats at different points in time was used to calculate their correlation coefficients. RESULTS: A total of 14 constituents were observed in the RSGE-treated rat serum, and 11 of these were inferred. An RSGE constituent was considered correlated with the hypouricemic effects if its correlation coefficient was above 0.5. The results suggested that only seven of the constituents absorbed in the serum of the hyperuricemic rats were correlated with hypouricemic effects, namely, palmitic acid, 3'-O-methyltaxifolin glucuronide, 3'-O-methyiastilbin glucuronide, astilbin glucuronide, 5-O-caffeoylshikimic acid glucuronide, resveratrol glucuronide, and dihydrokaempferol. CONCLUSION: These findings provide potent evidence for the study on RSG as a pharmacodynamic material basis and for developing RSG as a safe and promising natural drug to prevent hyperuricemia and gout instead of allopurinol.
Effect of rosuvastatin on hyperuricemic rats and the protective effect on endothelial dysfunction.[Pubmed:25371715]
Exp Ther Med. 2014 Dec;8(6):1683-1688.
Endothelial dysfunction plays a key role in the development of cardiovascular diseases, renal injuries and hypertension induced by hyperuricemia. Therapies targeting uric acid (UA) may be beneficial in cardiovascular diseases. In the present study, the effect of rosuvastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, was investigated to determine whether rosuvastatin improves endothelial dysfunction via the endothelial nitric oxide (NO) pathway and delays the pathogenesis of endothelial dysfunction in hyperuricemic rats. A total of 72 Sprague-Dawley rats (age, 8 weeks) were randomly divided into six groups (12 rats per group), including the control, model, 2.5 mg/kg/day rosuvastatin, 5 mg/kg/day rosuvastatin, 10 mg/kg/day rosuvastatin and 53.57 mg/kg/day allopurinol groups. The model, rosuvastatin and allopurinol rats were subjected to hyperuricemia, induced by the administration of yeast extract powder (21 g/kg/day) and Oxonic acid potassium salt (200 mg/kg/day). The hyperuricemic rats were treated with 2.5, 5.0 or 10.0 mg/kg/day rosuvastatin orally for six weeks, while rats treated with allopurinol (53.57 mg/kg/day) were used as a positive control. The serum levels of NO and the gene expression levels of endothelial NO synthase in the aortic tissue increased, whereas the serum levels of UA, endothelin-1 and angiotensin II decreased in the hyperuricemic rats treated with rosuvastatin, particularly at a high rosuvastatin dose (10 mg/kg/day). In addition, the curative effect of the 10 mg/kg/day rosuvastatin group was evidently higher compared with the allopurinol group. Therefore, rosuvastatin may be a novel drug candidate for the treatment of hyperuricemia due to its endothelial protective properties.
[A 13-week oral repeated dose toxicity study of a new antineoplastic agent S-1 in rats].[Pubmed:9021659]
J Toxicol Sci. 1996 Nov;21 Suppl 3:505-26.
S-1, an antineoplastic formulation of a fluorinated pyrimidine derivative containing tegafur (FT), CDHP, and potassium oxonate (Oxo) in a molar ratio of 1:0.4:1, was recently developed by Taiho Pharmaceutical Co., Ltd., with the aim of prolonging the effective plasma concentration of 5-fluorouracil (5-FU) over that produced by FT alone and reducing its dose-limiting gastrointestinal toxicity. As a part of the S-1 toxicity study, a 13-week oral repeated dose toxicity study and a recovery study using male and female rats was conducted. Doses of S-1 were adjusted to deliver 1.5, 5, and 15 mg/kg/day as doses of FT, and FT was given at 15 mg/kg/day. The following results were obtained. 1. In clinical observation, edema of the limbs and face or swelling of the auricle of the ear and an anemic appearance were observed in both sexes in the 15 mg/kg/day group as dose of FT. Subsequently, males in this group developed severe anemia, decreased spontaneous motor activity, emaciation, and subnormal skin temperature, and many males died. In the survivors, keratosis of the palm, sole, or tail was observed, with necrosis and loss of the tail tip in the severe cases. 2. Body weight gain was suppressed from about week 2 of treatment in both sexes in the 15 mg/kg/day group as dose of FT, and there was almost no weight gain after week 4-5. Food consumption was consistently less than the control value for males in the 15 mg/kg/day group as dose of FT throughout the treatment period. 3. No marked changes were observed in water intake and on opthalmologic examination. 4. In the fecal test for occult blood, a positive tendency was observed in both sexes in the 15 mg/kg/day group as dose of FT. 5. Urinalysis disclosed a slight increase in protein and decrease in sodium, potassium, and chloride in males, and an increase in protein in females in the 15 mg/kg/day group as dose of FT. 6. Hematologically, both sexes in the 15 mg/kg/day group as dose of FT showed decreases in red blood cell count, hemoglobin, and hematocrit, and increases in platelet count and fibrinogen, with a slight decrease in white blood cell count in males. 7. In the blood biochemical test, abnormal findings included increases in total cholesterol and free cholesterol, and decreases in non-esterified fatty acid and albumin in both sexes in the 15 mg/kg/day group as dose of FT. 8. In organ weight measurement, abnormal changes included a decrease in thymus weight in both sexes in the 5 mg/kg/day or higher dosage groups and a decrease in the testis weight in males and an increase in the liver weight in females in the 15 mg/kg/day group as dose of FT. 9. Histopathologically, both sexes in the 15 mg/kg/day group as dose of FT showed a decrease in the red pulp of the bone marrow, atrophy of the thymus, white pulp of the spleen, and testes. degeneration of the renal tubules, and ulcerative changes of the skin or oral mucosa. 10. The findings were unremarkable in the FT group. 11. During the recovery study, all the toxic effects tended to reverse. 12. The NOAEL of S-1 was estimated to be 1.5 mg/kg/day as dose of FT for both sexes.
Miracle Fruit (Synsepalum dulcificum) Exhibits as a Novel Anti-Hyperuricaemia Agent.[Pubmed:26821007]
Molecules. 2016 Jan 26;21(2):140.
Miracle fruit (Synsepalum dulcificum) belongs to the Sapotaceae family. It can change flavors on taste buds, transforming acidic tastes to sweet. We evaluated various miracle fruit extracts, including water, butanol, ethyl acetate (EA), and hexane fractions, to determine its antioxidant effects. These extracts isolated from miracle fruit exerted potential for reduction of uric acid and inhibited xanthine oxidase activity in vitro and in monosodiumurate (MSU)-treated RAW264.7 macrophages. Moreover, we also found that the butanol extracts of miracle fruit attenuated Oxonic acid potassium salt-induced hyperuricaemia in ICR mice by lowering serum uric acid levels and activating hepatic xanthine oxidase. These effects were equal to those of allopurinol, suggesting that the butanol extract of miracle fruit could be developed as a novel anti-hyperuricaemia agent or health food.
Strengthening the stability of a tunnel-shaped homotetramer protein with nanogels.[Pubmed:21699160]
J Phys Chem B. 2011 Jul 21;115(28):8875-82.
Urate oxidase (UOX, EC 1.7.3.3) is effective for the treatment of gout and hyperuricaemia associated with tumor lysis syndrome. The inherent poor stability of UOX to temperature, proteolysis, and acidic environments is known to limit its efficacy. Herein, we encapsulated UOX into spherical and porous nanogels with diameters of 20-40 nm via a two-step in situ polymerization in the presence of Oxonic acid potassium salt, an inhibitor of UOX. The UOX nanogel retained 70% of the initial activity but showed an expanded pH spectrum from pH 6-10 to 3-10 and an extended half-life at 37 degrees C from 5 min to 3 h. The enhanced pH stability, thermal stability, and enzyme resistance of the UOX nanogels were also confirmed by using fluorescence spectroscopy and enzymatic digestion. A molecular dynamics simulation was performed as a way to probe the mechanism underlying the formation of UOX nanogels as well as the strengthened stability against harsh conditions. It was shown that the encapsulation into the polyacrylamide network reinforced the intersubunit hydrogen bonding, shielded the hydrolytic reaction site, and thus protected the tertiary and quaternary structure of UOX. The UOX nanogel with enhanced stability provided a stable enzyme model that enables the exploration of UOX in the diagnosis and therapy of disorders associated with altered purine metabolism.


