ThunalbeneCAS# 220862-05-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 220862-05-5 | SDF | Download SDF |
| PubChem ID | 25756094 | Appearance | Powder |
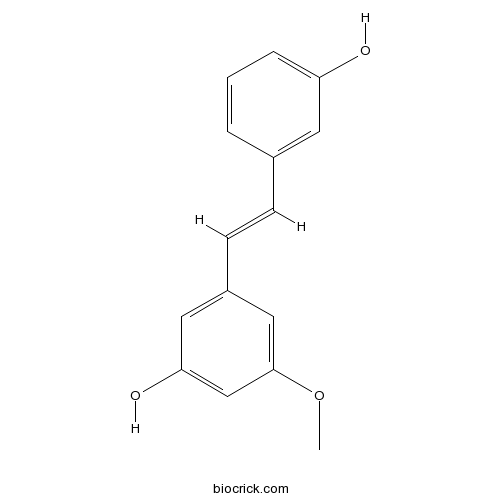
| Formula | C15H14O3 | M.Wt | 242.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-[(E)-2-(3-hydroxyphenyl)ethenyl]-5-methoxyphenol | ||
| SMILES | COC1=CC(=CC(=C1)O)C=CC2=CC(=CC=C2)O | ||
| Standard InChIKey | VANIIUGEHGLNHB-AATRIKPKSA-N | ||
| Standard InChI | InChI=1S/C15H14O3/c1-18-15-9-12(8-14(17)10-15)6-5-11-3-2-4-13(16)7-11/h2-10,16-17H,1H3/b6-5+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Thunalbene shows weak inhibitory activities without cytotoxicity on the production of nitric oxide (NO) which is an important inflammatory mediator. |
| Targets | NO |

Thunalbene Dilution Calculator

Thunalbene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1271 mL | 20.6356 mL | 41.2712 mL | 82.5423 mL | 103.1779 mL |
| 5 mM | 0.8254 mL | 4.1271 mL | 8.2542 mL | 16.5085 mL | 20.6356 mL |
| 10 mM | 0.4127 mL | 2.0636 mL | 4.1271 mL | 8.2542 mL | 10.3178 mL |
| 50 mM | 0.0825 mL | 0.4127 mL | 0.8254 mL | 1.6508 mL | 2.0636 mL |
| 100 mM | 0.0413 mL | 0.2064 mL | 0.4127 mL | 0.8254 mL | 1.0318 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7-Methoxy-8-Hydroxy-4-Methylcoumarin
Catalog No.:BCC8291
CAS No.:22084-94-2
- NG,NG-Dimethylarginine dihydrochloride
Catalog No.:BCN1485
CAS No.:220805-22-1
- Aminopurvalanol A
Catalog No.:BCC7249
CAS No.:220792-57-4
- Aquifoliunine E-III
Catalog No.:BCN3096
CAS No.:220751-20-2
- Curlignan
Catalog No.:BCN3977
CAS No.:220736-54-9
- Ketoprofen
Catalog No.:BCC4430
CAS No.:22071-15-4
- Oxonic acid potassium salt
Catalog No.:BCC4165
CAS No.:2207-75-2
- Antisauvagine-30
Catalog No.:BCC5868
CAS No.:220673-95-0
- RJR-2403 oxalate
Catalog No.:BCC1902
CAS No.:220662-95-3
- Tigecycline
Catalog No.:BCC2499
CAS No.:220620-09-7
- GB 1a
Catalog No.:BCN7237
CAS No.:220611-41-6
- Diphyllin
Catalog No.:BCN8066
CAS No.:22055-22-7
- 2,3-O-Isopropylidenyl euscaphic acid
Catalog No.:BCN4944
CAS No.:220880-90-0
- GW5074
Catalog No.:BCC4391
CAS No.:220904-83-6
- 13-O-Cinnamoylbaccatin III
Catalog No.:BCN7344
CAS No.:220932-65-0
- 13-O-Deacetyltaxumairol Z
Catalog No.:BCN4945
CAS No.:220935-39-7
- Antalarmin hydrochloride
Catalog No.:BCC7480
CAS No.:220953-69-5
- Lumiracoxib
Catalog No.:BCC4440
CAS No.:220991-20-8
- Guaiacol glycidyl ether
Catalog No.:BCC8992
CAS No.:2210-74-4
- 1''-Methoxyerythrinin C
Catalog No.:BCN3966
CAS No.:221002-11-5
- 1alpha,4beta,10beta-Trihydroxyguaia-2,11(13)-dien-12,6alpha-olide
Catalog No.:BCN7483
CAS No.:221148-94-3
- Erysubin A
Catalog No.:BCN4946
CAS No.:221150-18-1
- Erysubin B
Catalog No.:BCN4947
CAS No.:221150-19-2
- Glyoxalase I inhibitor
Catalog No.:BCC1598
CAS No.:221174-33-0
Metabolism of Stilbenoids by Human Faecal Microbiota.[Pubmed:30909544]
Molecules. 2019 Mar 23;24(6). pii: molecules24061155.
Stilbenoids are dietary phenolics with notable biological effects on humans. Epidemiological, clinical, and nutritional studies from recent years have confirmed the significant biological effects of stilbenoids, such as oxidative stress protection and the prevention of degenerative diseases, including cancer, cardiovascular diseases, and neurodegenerative diseases. Stilbenoids are intensively metabolically transformed by colon microbiota, and their corresponding metabolites might show different or stronger biological activity than their parent molecules. The aim of the present study was to determine the metabolism of six stilbenoids (resveratrol, oxyresveratrol, piceatannol, Thunalbene, batatasin III, and pinostilbene), mediated by colon microbiota. Stilbenoids were fermented in an in vitro faecal fermentation system using fresh faeces from five different donors as an inoculum. The samples of metabolized stilbenoids were collected at 0, 2, 4, 8, 24, and 48 h. Significant differences in the microbial transformation among stilbene derivatives were observed by liquid chromatography mass spectrometry (LC/MS). Four stilbenoids (resveratrol, oxyresveratrol, piceatannol and Thunalbene) were metabolically transformed by double bond reduction, dihydroxylation, and demethylation, while batatasin III and pinostilbene were stable under conditions simulating the colon environment. Strong inter-individual differences in speed, intensity, and pathways of metabolism were observed among the faecal samples obtained from the donors.
Effect of Selected Stilbenoids on Human Fecal Microbiota.[Pubmed:30791436]
Molecules. 2019 Feb 19;24(4). pii: molecules24040744.
Dietary phenolics or polyphenols are mostly metabolized by the human gut microbiota. These metabolites appear to confer the beneficial health effects attributed to phenolics. Microbial composition affects the type of metabolites produced. Reciprocally, phenolics modulate microbial composition. Understanding this relationship could be used to positively impact health by phenolic supplementation and thus create favorable colonic conditions. This study explored the effect of six stilbenoids (batatasin III, oxyresveratrol, piceatannol, pinostilbene, resveratrol, Thunalbene) on the gut microbiota composition. Stilbenoids were anaerobically fermented with fecal bacteria from four donors, samples were collected at 0 and 24 h, and effects on the microbiota were assessed by 16S rRNA gene sequencing. Statistical tests identified affected microbes at three taxonomic levels. Observed microbial composition modulation by stilbenoids included a decrease in the Firmicutes to Bacteroidetes ratio, a decrease in the relative abundance of strains from the genus Clostridium, and effects on the family Lachnospiraceae. A frequently observed effect was a further decrease of the relative abundance when compared to the control. An opposite effect to the control was observed for Faecalibacterium prausnitzii, whose relative abundance increased. Observed effects were more frequently attributed to resveratrol and piceatannol, followed by Thunalbene and batatasin III.


