Glyoxalase I inhibitorGlyoxalase I inhibitor CAS# 221174-33-0 |
- PDK1 inhibitor
Catalog No.:BCC1843
CAS No.:1001409-50-2
- Nemorubicin
Catalog No.:BCC4151
CAS No.:108852-90-0
- Siramesine hydrochloride
Catalog No.:BCC5134
CAS No.:224177-60-0
- Oleuropein
Catalog No.:BCN5246
CAS No.:32619-42-4
- Fluorouracil (Adrucil)
Catalog No.:BCC2135
CAS No.:51-21-8
- Deguelin
Catalog No.:BCN4804
CAS No.:522-17-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 221174-33-0 | SDF | Download SDF |
| PubChem ID | 66577013 | Appearance | Powder |
| Formula | C21H30BrClN4O8S | M.Wt | 613.91 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 37 mg/mL (60.27 mM) *"≥" means soluble, but saturation unknown. | ||
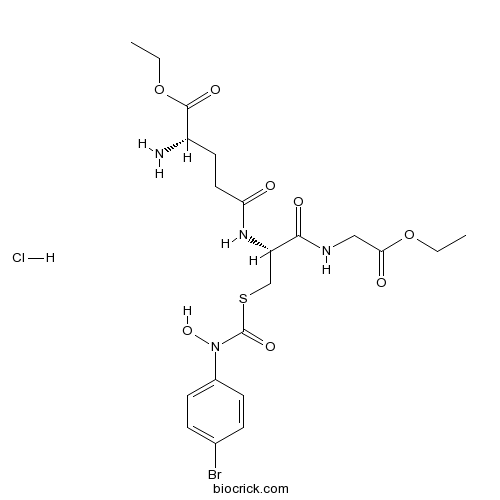
| Chemical Name | ethyl (2S)-2-amino-5-[[(2R)-3-[(4-bromophenyl)-hydroxycarbamoyl]sulfanyl-1-[(2-ethoxy-2-oxoethyl)amino]-1-oxopropan-2-yl]amino]-5-oxopentanoate;hydrochloride | ||
| SMILES | CCOC(=O)CNC(=O)C(CSC(=O)N(C1=CC=C(C=C1)Br)O)NC(=O)CCC(C(=O)OCC)N.Cl | ||
| Standard InChIKey | HFQRZRCYYQCUIG-MOGJOVFKSA-N | ||
| Standard InChI | InChI=1S/C21H29BrN4O8S.ClH/c1-3-33-18(28)11-24-19(29)16(25-17(27)10-9-15(23)20(30)34-4-2)12-35-21(31)26(32)14-7-5-13(22)6-8-14;/h5-8,15-16,32H,3-4,9-12,23H2,1-2H3,(H,24,29)(H,25,27);1H/t15-,16-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Glyoxalase I inhibitor is a potent inhibitor of glyoxalase I. | |||||
| Targets | glyoxalase I | |||||

Glyoxalase I inhibitor Dilution Calculator

Glyoxalase I inhibitor Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6289 mL | 8.1445 mL | 16.289 mL | 32.5781 mL | 40.7226 mL |
| 5 mM | 0.3258 mL | 1.6289 mL | 3.2578 mL | 6.5156 mL | 8.1445 mL |
| 10 mM | 0.1629 mL | 0.8145 mL | 1.6289 mL | 3.2578 mL | 4.0723 mL |
| 50 mM | 0.0326 mL | 0.1629 mL | 0.3258 mL | 0.6516 mL | 0.8145 mL |
| 100 mM | 0.0163 mL | 0.0814 mL | 0.1629 mL | 0.3258 mL | 0.4072 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
S-(N-Aryl-N-hydroxycarbamoyl)glutathione derivatives have been proposed as possible anticancer agents, because of their ability to strongly inhibit the methylglyoxal-detoxifying enzyme glyoxalase I. Glyoxalase I inhibitor is a potent inhibitor of glyoxalase I.
In vitro: As a tumor-selective anticancer agent, Glyoxalase I inhibitor [3(Et)2] was evaluated against B16 melanotic melanoma, L1210 murine leukemia, and nonproliferating murine splenic lymphocytes in culture. The diethyl ester prodrugs of Glyoxalase I inhibitor [3(Et)2] also displayed significant tumour-selective toxicity towards L1210 cells compared with normal murine splenic lymphocytes in vitro [1].
In vivo: Small-scale efficacy studies indicated that 3b(Et)2 could effectively inhibit tumour growth in plasma esterase-deficient mice bearing murine B16 melanoma and in esterasedeficient athymic nude mice bearing androgen-independent human prostate PC-3 tumours or human colon HT-29 tumours [2].
Clinical trial: No clinical data are available.
References:
[1] Kavarana MJ, Kovaleva EG, Creighton DJ, Wollman MB, Eiseman JL. Mechanism-based competitive inhibitors of glyoxalase I: intracellular delivery, in vitro antitumor activities, and stabilities in human serum and mouse serum. J Med Chem. 1999;42(2):221-8.
[2] Creighton DJ, Zheng ZB, Holewinski R, Hamilton DS, Eiseman JL. Glyoxalase I inhibitors in cancer chemotherapy. Biochem Soc Trans. 2003;31(Pt 6):1378-82.
- Erysubin B
Catalog No.:BCN4947
CAS No.:221150-19-2
- Erysubin A
Catalog No.:BCN4946
CAS No.:221150-18-1
- 1alpha,4beta,10beta-Trihydroxyguaia-2,11(13)-dien-12,6alpha-olide
Catalog No.:BCN7483
CAS No.:221148-94-3
- 1''-Methoxyerythrinin C
Catalog No.:BCN3966
CAS No.:221002-11-5
- Guaiacol glycidyl ether
Catalog No.:BCC8992
CAS No.:2210-74-4
- Lumiracoxib
Catalog No.:BCC4440
CAS No.:220991-20-8
- Antalarmin hydrochloride
Catalog No.:BCC7480
CAS No.:220953-69-5
- 13-O-Deacetyltaxumairol Z
Catalog No.:BCN4945
CAS No.:220935-39-7
- 13-O-Cinnamoylbaccatin III
Catalog No.:BCN7344
CAS No.:220932-65-0
- GW5074
Catalog No.:BCC4391
CAS No.:220904-83-6
- 2,3-O-Isopropylidenyl euscaphic acid
Catalog No.:BCN4944
CAS No.:220880-90-0
- Thunalbene
Catalog No.:BCN3688
CAS No.:220862-05-5
- Z-Lys-OH
Catalog No.:BCC2764
CAS No.:2212-75-1
- Z-Lys(Boc)-OH.DCHA
Catalog No.:BCC2591
CAS No.:2212-76-2
- 1-Naphthyl PP1
Catalog No.:BCC3893
CAS No.:221243-82-9
- 1-NM-PP1
Catalog No.:BCC4306
CAS No.:221244-14-0
- 7,4'-Di-O-methylapigenin 5-O-xylosylglucoside
Catalog No.:BCN1484
CAS No.:221257-06-3
- Lethedoside A
Catalog No.:BCN4948
CAS No.:221289-20-9
- Lethedioside A
Catalog No.:BCN5046
CAS No.:221289-31-2
- Leptocarpinine
Catalog No.:BCN3730
CAS No.:221347-12-2
- Podocarpusflavone A
Catalog No.:BCN5047
CAS No.:22136-74-9
- Pinosylvin
Catalog No.:BCN5048
CAS No.:22139-77-1
- Cytochalasin D
Catalog No.:BCN5049
CAS No.:22144-77-0
- Fischeria A
Catalog No.:BCN3779
CAS No.:221456-63-9
TLSC702, a Novel Inhibitor of Human Glyoxalase I, Induces Apoptosis in Tumor Cells.[Pubmed:27150153]
Biol Pharm Bull. 2016;39(5):869-73.
Human glyoxalase I (hGLO I) is a rate-limiting enzyme in the pathway for detoxification of apoptosis-inducible methylglyoxal (MG), which is the side product of tumor-specific aerobic glycolysis. GLO I has been reported to be overexpressed in various types of cancer cells, and has been expected as an attractive target for the development of new anticancer drugs. We previously discovered a novel inhibitor of hGLO I, named TLSC702, by our in silico screening method. Here, we show that TLSC702 inhibits the proliferation of human leukemia HL-60 cells and induces apoptosis in a dose-dependent manner. In addition, TLSC702 more significantly inhibits the proliferation of human lung cancer NCI-H522 cells, which highly express GLO I, than that of GLO I lower-expressing human lung cancer NCI-H460 cells. Furthermore, this antiproliferative effect of TLSC702 on NCI-H522 cells is in a dose- and time-dependent manner. These results suggest that TLSC702 can induce apoptosis in tumor cells by GLO I inhibition, which lead to accumulation of MG. Taken together, TLSC702 could become a unique seed compound for the generation of novel chemotherapeutic drugs targeting GLO I-dependent human tumors.
Synthesis of azide derivative and discovery of glyoxalase pathway inhibitor against pathogenic bacteria.[Pubmed:24076169]
Bioorg Med Chem Lett. 2013 Nov 15;23(22):6138-40.
A glyoxalase inhibitor was synthesized and tested against Staphylococcus aureus for first time and showed MIC90 of 20 mug/ml. Henceforth, we synthesized unnatural azide derivative of the same inhibitor to improve the biological activity. In that order, an azide carboxylate was synthesized from dimethyl tartrate by tosylation and azide substitution. The synthesized, azide compound was coupled with glutathione derivative in high yield and tested against S. aureus and showed improved MIC90 of 5 mug/ml. In general, it can be also easily converted to unnatural beta-amino acid in good yield. The shown methodology will be extended to study induced suicide in Burkholderia mallei, Francisella tularensis and Mycobacterium tuberculosis in future.
Structural basis for 18-beta-glycyrrhetinic acid as a novel non-GSH analog glyoxalase I inhibitor.[Pubmed:26279158]
Acta Pharmacol Sin. 2015 Sep;36(9):1145-50.
AIM: Glyoxalase I (GLOI), a glutathione (GSH)-dependent enzyme, is overexpressed in tumor cells and related to multi-drug resistance in chemotherapy, making GLOI inhibitors as potential anti-tumor agents. But the most studied GSH analogs exhibit poor pharmacokinetic properties. The aim of this study was to discover novel non-GSH analog GLOI inhibitors and analyze their binding mechanisms. METHODS: Mouse GLOI (mGLOI) was expressed in BL21 (DE3) pLysS after induction with isopropyl-beta-D-1-thiogalactopyranoside and purified using AKTA FPLC system. An in vitro mGLOI enzyme assay was used to screen a small pool of compounds containing carboxyl groups. Crystal structure of the mGLOI-inhibitor complex was determined at 2.3 A resolution. Molecular docking study was performed using Discovery Studio 2.5 software package. RESULTS: A natural compound 18-beta-glycyrrhetinic acid (GA) and its derivative carbenoxolone were identified as potent competitive non-GSH analog mGLOI inhibitors with Ki values of 0.29 mumol/L and 0.93 mumol/L, respectively. Four pentacyclic triterpenes (ursolic acid, oleanolic acid, betulic acid and tripterine) showed weak activities (mGLOI inhibition ratio <25% at 10 mumol/L) and other three (maslinic acid, corosolic acid and madecassic acid) were inactive. The crystal structure of the mGLOI-GA complex showed that the carboxyl group of GA mimicked the gamma-glutamyl residue of GSH by hydrogen bonding to the glutamyl sites (residues Arg38B, Asn104B and Arg123A) in the GSH binding site of mGLOI. The extensive van der Waals interactions between GA and the surrounding residues also contributed greatly to the binding of GA and mGLOI. CONCLUSION: This work demonstrates a carboxyl group to be an important functional feature of non-GSH analog GLOI inhibitors.


