PinosylvinCAS# 22139-77-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 22139-77-1 | SDF | Download SDF |
| PubChem ID | 5280457 | Appearance | White-beige powder |
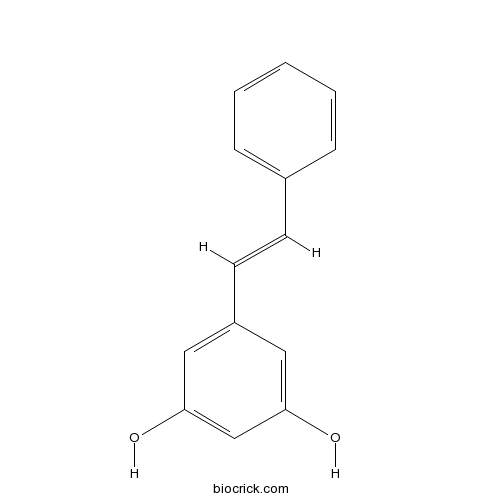
| Formula | C14H12O2 | M.Wt | 212.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | trans-3,5-Dihydroxystilbene; (E)-3,5-Stilbenediol | ||
| Solubility | Soluble in methan | ||
| Chemical Name | 5-[(E)-2-phenylethenyl]benzene-1,3-diol | ||
| SMILES | C1=CC=C(C=C1)C=CC2=CC(=CC(=C2)O)O | ||
| Standard InChIKey | YCVPRTHEGLPYPB-VOTSOKGWSA-N | ||
| Standard InChI | InChI=1S/C14H12O2/c15-13-8-12(9-14(16)10-13)7-6-11-4-2-1-3-5-11/h1-10,15-16H/b7-6+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pinosylvin is likely to act as a pro-angiogenic factor, it has anti-inflammatory activity, it may be utilized as a phytotherapic agent for the prevention of cardiovascular inflammatory diseases. Pinosylvin is an effective inhibitor of neutrophil activity, and is potentially useful as a complementary medicine in states associated with persistent inflammation. Pinosylvin has protection against oxidative stress through the induction of HO-1 in human RPE cells. Pinosylvin has inhibition against White-Rot and Brown-Rot Fungi. |
| Targets | AMPK | HO-1 | Caspase | MMP(e.g.TIMP) | COX | ERK | Akt | NOS | NO | IL Receptor | PKC | Antifection | Autophagy |
| In vitro | Pinosylvin at a high concentration induces AMPK-mediated autophagy for preventing necrosis in bovine aortic endothelial cells.[Pubmed: 25393712 ]Can J Physiol Pharmacol. 2014 Dec;92(12):993-9.Pinosylvin is a known functional compound of the Pinus species. Pinosylvin at low concentrations (∼ pmol/L) was reported to promote cell proliferation in endothelial cells. However, this study found that Pinosylvin at a high concentration (100 μmol/L) induces cell death in bovine aortic endothelial cells. Therefore, we examined how Pinosylvin was associated with apoptosis, autophagy, and necrosis. Antimetastatic activity of pinosylvin, a natural stilbenoid, is associated with the suppression of matrix metalloproteinases.[Pubmed: 21937212 ]J Nutr Biochem. 2012 Aug;23(8):946-52.Metastasis is a major cause of death in cancer patients. Our previous studies showed that Pinosylvin, a naturally occurring trans-stilbenoid mainly found in Pinus species, exhibited a potential cancer chemopreventive activity and also inhibited the growth of various human cancer cell lines via the regulation of cell cycle progression. Efficacy of Pinosylvins against White-Rot and Brown-Rot Fungi.[Reference: WebLink]Holzforschung, 1999, 53(5):491-7.
|
| In vivo | Pinosylvin and Monomethylpinosylvin, Constituents of an Extract from the Knot of Pinus sylvestris, Reduce Inflammatory Gene Expression and Inflammatory Responses in Vivo.[Pubmed: 25763469]J Agric Food Chem. 2015 Apr 8;63(13):3445-53.Scots pine (Pinus sylvestris) is known to be rich in phenolic compounds, which may have anti-inflammatory properties. |
| Kinase Assay | Pinosylvin induces cell survival, migration and anti-adhesiveness of endothelial cells via nitric oxide production.[Pubmed: 22736379]Phytother Res. 2013 Apr;27(4):610-7.Pinosylvin is a phenolic compound mainly found in the Pinus species. To determine the vascular functions of Pinosylvin, we first examined both proliferation and apoptosis of bovine aortic endothelial cells (BAECs) in the presence of Pinosylvin. |
| Animal Research | The natural stilbenoid pinosylvin and activated neutrophils: effects on oxidative burst, protein kinase C, apoptosis and efficiency in adjuvant arthritis.[Pubmed: 22842731 ]Acta Pharmacol Sin. 2012 Oct;33(10):1285-92.To investigate the effects of the naturally occurring stilbenoid Pinosylvin on neutrophil activity in vitro and in experimental arthritis, and to examine whether protein kinase C (PKC) activation served as an assumed target of Pinosylvin action.
|

Pinosylvin Dilution Calculator

Pinosylvin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.7103 mL | 23.5516 mL | 47.1032 mL | 94.2063 mL | 117.7579 mL |
| 5 mM | 0.9421 mL | 4.7103 mL | 9.4206 mL | 18.8413 mL | 23.5516 mL |
| 10 mM | 0.471 mL | 2.3552 mL | 4.7103 mL | 9.4206 mL | 11.7758 mL |
| 50 mM | 0.0942 mL | 0.471 mL | 0.9421 mL | 1.8841 mL | 2.3552 mL |
| 100 mM | 0.0471 mL | 0.2355 mL | 0.471 mL | 0.9421 mL | 1.1776 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Podocarpusflavone A
Catalog No.:BCN5047
CAS No.:22136-74-9
- Leptocarpinine
Catalog No.:BCN3730
CAS No.:221347-12-2
- Lethedioside A
Catalog No.:BCN5046
CAS No.:221289-31-2
- Lethedoside A
Catalog No.:BCN4948
CAS No.:221289-20-9
- 7,4'-Di-O-methylapigenin 5-O-xylosylglucoside
Catalog No.:BCN1484
CAS No.:221257-06-3
- 1-NM-PP1
Catalog No.:BCC4306
CAS No.:221244-14-0
- 1-Naphthyl PP1
Catalog No.:BCC3893
CAS No.:221243-82-9
- Z-Lys(Boc)-OH.DCHA
Catalog No.:BCC2591
CAS No.:2212-76-2
- Z-Lys-OH
Catalog No.:BCC2764
CAS No.:2212-75-1
- Glyoxalase I inhibitor
Catalog No.:BCC1598
CAS No.:221174-33-0
- Erysubin B
Catalog No.:BCN4947
CAS No.:221150-19-2
- Erysubin A
Catalog No.:BCN4946
CAS No.:221150-18-1
- Cytochalasin D
Catalog No.:BCN5049
CAS No.:22144-77-0
- Fischeria A
Catalog No.:BCN3779
CAS No.:221456-63-9
- 2-Oxokolavelool
Catalog No.:BCN4672
CAS No.:221466-41-7
- 2 beta-Hydroxykolavelool
Catalog No.:BCN4671
CAS No.:221466-42-8
- 11-Hydroxytabersonine
Catalog No.:BCN5050
CAS No.:22149-28-6
- Kaempferol 3-gentiobioside
Catalog No.:BCN3345
CAS No.:22149-35-5
- Hopane-3beta,22-diol
Catalog No.:BCN4852
CAS No.:22149-65-1
- Stigmastane-3,6-dione
Catalog No.:BCN5051
CAS No.:22149-69-5
- 1-Benzyl-1H-indazol-3-ol
Catalog No.:BCC8458
CAS No.:2215-63-6
- 2,6-Dimethoxy-1-acetonylquinol
Catalog No.:BCN5052
CAS No.:2215-96-5
- Boron tributoxide
Catalog No.:BCN8281
CAS No.:688-74-4
- L-Menthol
Catalog No.:BCN4971
CAS No.:2216-51-5
The natural stilbenoid pinosylvin and activated neutrophils: effects on oxidative burst, protein kinase C, apoptosis and efficiency in adjuvant arthritis.[Pubmed:22842731]
Acta Pharmacol Sin. 2012 Oct;33(10):1285-92.
AIM: To investigate the effects of the naturally occurring stilbenoid Pinosylvin on neutrophil activity in vitro and in experimental arthritis, and to examine whether protein kinase C (PKC) activation served as an assumed target of Pinosylvin action. METHODS: Fresh human blood neutrophils were isolated. The oxidative burst of neutrophils was evaluated on the basis of enhanced chemiluminescence. Neutrophil viability was evaluated with flow cytometry, and PKC phosphorylation was assessed by Western blotting analysis. Adjuvant arthritis was induced in Lewis rats with heat-killed Mycobacterium butyricum, and the animals were administered with Pinosylvin (30 mg/kg, po) daily for 21 d after arthritis induction. RESULTS: In isolated human neutrophils, Pinosylvin (10 and 100 mumol/L) significantly decreased the formation of oxidants, both extra- and intracellularly, and effectively inhibited PKC activation stimulated by phorbol myristate acetate (0.05 mumol/L). The inhibition was not due to neutrophil damage or increased apoptosis. In arthritic rats, the number of neutrophils in blood was dramatically increased, and whole blood chemiluminescence (spontaneous and PMA-stimulated) was markedly enhanced. Pinosylvin administration decreased the number of neutrophils (from 69 671 +/- 5588/muL to 51 293 +/- 3947/muL, P=0.0198) and significantly reduced the amount of reactive oxygen species in blood. CONCLUSION: Pinosylvin is an effective inhibitor of neutrophil activity, and is potentially useful as a complementary medicine in states associated with persistent inflammation.
Pinosylvin at a high concentration induces AMPK-mediated autophagy for preventing necrosis in bovine aortic endothelial cells.[Pubmed:25393712]
Can J Physiol Pharmacol. 2014 Dec;92(12):993-9.
Pinosylvin is a known functional compound of the Pinus species. Pinosylvin at low concentrations ( approximately pmol/L) was reported to promote cell proliferation in endothelial cells. However, this study found that Pinosylvin at a high concentration (100 mumol/L) induces cell death in bovine aortic endothelial cells. Therefore, we examined how Pinosylvin was associated with apoptosis, autophagy, and necrosis. Pinosylvin at a high concentration appeared to promote caspase-3 activation, nuclear condensation, and the "flip-flop" of phosphatidylserine, indicating that Pinosylvin induces apoptosis. However, based on flow cytometry data obtained from double-staining with annexin V and propidium iodide, Pinosylvin was shown to inhibit necrosis, a postapoptotic process. Pinosylvin induced LC3 conversion from LC3-I to LC3-II and p62 degradation, which are important indicators of autophagy. In addition, AMP-activated protein kinase (AMPK) appeared to be activated by Pinosylvin, and an AMPK inhibitor was markedly shown to reduce the LC3 conversion. The inhibitory effect of an AMPK inhibitor was reversed by Pinosylvin. These results suggest that Pinosylvin induces autophagy via AMPK activation. Further, necrosis was found to be promoted by an autophagy inhibitor and then restored by Pinosylvin, while the caspase-3 inhibitor had no effect on necrosis. These findings indicate that Pinosylvin-induced autophagy blocks necrotic progress in endothelial cells.
Antimetastatic activity of pinosylvin, a natural stilbenoid, is associated with the suppression of matrix metalloproteinases.[Pubmed:21937212]
J Nutr Biochem. 2012 Aug;23(8):946-52.
Metastasis is a major cause of death in cancer patients. Our previous studies showed that Pinosylvin, a naturally occurring trans-stilbenoid mainly found in Pinus species, exhibited a potential cancer chemopreventive activity and also inhibited the growth of various human cancer cell lines via the regulation of cell cycle progression. In this study, we further evaluated the potential antimetastatic activity of Pinosylvin in in vitro and in vivo models. Pinosylvin suppressed the expression of matrix metalloproteinase (MMP)-2, MMP-9 and membrane type 1-MMP in cultured human fibrosarcoma HT1080 cells. We also found that Pinosylvin inhibited the migration of HT1080 cells in colony dispersion and wound healing assay systems. In in vivo spontaneous pulmonary metastasis model employing intravenously injected CT26 mouse colon cancer cells in Balb/c mice, Pinosylvin (10 mg/kg body weight, intraperitoneal administration) significantly inhibited the formation of tumor nodules and tumor weight in lung tissues. The analysis of tumor in lung tissues indicated that the antimetastatic effect of Pinosylvin coincided with the down-regulation of MMP-9 and cyclooxygenase-2 expression, and phosphorylation of ERK1/2 and Akt. These data suggest that Pinosylvin might be an effective inhibitor of tumor cell metastasis via modulation of MMPs.
Pinosylvin and monomethylpinosylvin, constituents of an extract from the knot of Pinus sylvestris, reduce inflammatory gene expression and inflammatory responses in vivo.[Pubmed:25763469]
J Agric Food Chem. 2015 Apr 8;63(13):3445-53.
Scots pine (Pinus sylvestris) is known to be rich in phenolic compounds, which may have anti-inflammatory properties. The present study investigated the anti-inflammatory effects of a knot extract from P. sylvestris and two stilbenes, Pinosylvin and monomethylPinosylvin, isolated from the extract. Inflammation is characterized by increased release of pro-inflammatory and regulatory mediators including nitric oxide (NO) produced by the inducible nitric oxide synthase (iNOS) pathway. The knot extract (EC50 values of 3 and 3 mug/mL) as well as two of its constituents, Pinosylvin (EC50 values of 13 and 15 muM) and monomethylPinosylvin (EC50 values of 8 and 12 muM), reduced NO production and iNOS expression in activated macrophages. They also inhibited the production of inflammatory cytokines IL-6 and MCP-1. More importantly, Pinosylvin and monomethylPinosylvin exerted a clear anti-inflammatory effect (80% inhibition at the dose of 100 mg/kg) in the standard in vivo model, carrageenan-induced paw inflammation in the mouse, with the effect being comparable to that of a known iNOS inhibitor L-NIL. The results reveal that the Scots pine stilbenes Pinosylvin and monomethylPinosylvin are potential anti-inflammatory compounds.
Pinosylvin induces cell survival, migration and anti-adhesiveness of endothelial cells via nitric oxide production.[Pubmed:22736379]
Phytother Res. 2013 Apr;27(4):610-7.
Pinosylvin is a phenolic compound mainly found in the Pinus species. To determine the vascular functions of Pinosylvin, we first examined both proliferation and apoptosis of bovine aortic endothelial cells (BAECs) in the presence of Pinosylvin. When BAECs were treated with Pinosylvin, etoposide- or starvation-induced apoptosis was shown to be significantly reduced. The anti-apoptotic effect of Pinosylvin was mediated by inhibition of caspase-3. Moreover, Pinosylvin was shown to activate endothelial nitric oxide synthetase (eNOS). At 1 pM, Pinosylvin appeared to have a cell-proliferative effect in the endothelial cell. The Pinosylvin-induced cell proliferation was declined by treatment with L-NAME, an eNOS inhibitor. Then, we found that Pinosylvin had a stimulatory effect on cell migration and tube formation. These stimulatory effects suggest that Pinosylvin is likely to act as a pro-angiogenic factor. Yet another effect of Pinosylvin was inhibition of lipopolysaccharide-induced THP-1 cell adhesion to endothelial cells. Altogether, we propose that Pinosylvin may be utilized as a phytotherapic agent for the prevention of cardiovascular inflammatory diseases.


