Cytochalasin Dinhibitor of actin polymerization, selective CAS# 22144-77-0 |
- Mithramycin A
Catalog No.:BCC2470
CAS No.:18378-89-7
- Foscarnet Sodium
Catalog No.:BCC4782
CAS No.:63585-09-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 22144-77-0 | SDF | Download SDF |
| PubChem ID | 5458428 | Appearance | Powder |
| Formula | C30H37NO6 | M.Wt | 507.6 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Zygosporin A | ||
| Solubility | Soluble to 10 mM in ethanol with gentle warming and to 50 mM in DMSO | ||
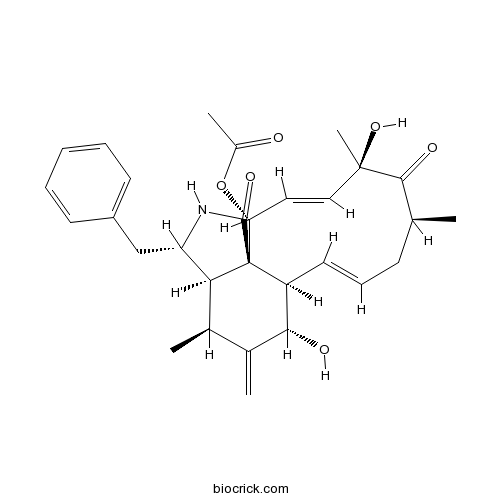
| SMILES | CC1CC=CC2C(C(=C)C(C3C2(C(C=CC(C1=O)(C)O)OC(=O)C)C(=O)NC3CC4=CC=CC=C4)C)O | ||
| Standard InChIKey | SDZRWUKZFQQKKV-JHADDHBZSA-N | ||
| Standard InChI | InChI=1S/C30H37NO6/c1-17-10-9-13-22-26(33)19(3)18(2)25-23(16-21-11-7-6-8-12-21)31-28(35)30(22,25)24(37-20(4)32)14-15-29(5,36)27(17)34/h6-9,11-15,17-18,22-26,33,36H,3,10,16H2,1-2,4-5H3,(H,31,35)/b13-9+,15-14+/t17-,18+,22-,23-,24+,25-,26+,29+,30+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Cytochalasin D-induced extension of neurite-like processes might correlate with enhanced accumulation of PrPres. 2. Cytochalasin D is an actin inhibitor, the removal of actin stress fibers is crucial for the chondrogenic differentiation. 3. Cytochalasin D inhibits CT26 tumor growth potentially through inhibition of cell proliferation, induction of cell apoptosis and suppression of tumor angiogenesis. 4. Cytochalasin D stimulates the expression of TF in B16 melanoma cells, activating both coagulation-dependent and -independent pathways via binding to FVIIa, eventually promoting lung metastasis. 5. Cytochalasin D inhibits smooth muscle contraction by directly inhibiting contractile apparatus. 6. Cytochalasin D is an inhibitor of microfilament-dependent phagocytosis, it (0.5 or 1.0 micrograms/ml) can inhibit intracellular multiplication of L. pneumophila in U937 monocytes. 7. Cytochalasin D may be an inhibitor of some fertilization processes such as sperm penetration or sperm head decondensation. |
| Targets | p38MAPK | PI3K | Akt | Calcium Channel | ATPase |

Cytochalasin D Dilution Calculator

Cytochalasin D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9701 mL | 9.8503 mL | 19.7006 mL | 39.4011 mL | 49.2514 mL |
| 5 mM | 0.394 mL | 1.9701 mL | 3.9401 mL | 7.8802 mL | 9.8503 mL |
| 10 mM | 0.197 mL | 0.985 mL | 1.9701 mL | 3.9401 mL | 4.9251 mL |
| 50 mM | 0.0394 mL | 0.197 mL | 0.394 mL | 0.788 mL | 0.985 mL |
| 100 mM | 0.0197 mL | 0.0985 mL | 0.197 mL | 0.394 mL | 0.4925 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cytochalasin D is a selective inhibitor of actin polymerization with with IC50 value of 25 nM [1].
Actin is a globular multi-functional protein and found nearly in all eukaryotic cells. it has been shown that actin polymerization plays a pivotal role in chemotaxis and cytokinesis. Cytochalasin D is reported as an inhibitor in the process of actin polymerization via disrupting actin microfilaments and activating p53-dependent pathways which in turn causes the arrest of cell cycle at the G1-S transition [2].
Cytochalasin D is a potent actin polymerization inhibitor. When tested with differentiating neurons, Cytochalasin D slowed down protrusion/retraction cycles and decreased lamellipodia axial motion via inhibiting actin polymerization [1]. In epithelial cell line HEp-2 cells, Cytochalasin D treatment regulated late and very late phases of viral transcription and shut down host transcription through blocking actin polymerization [3]. In the model of IPEC-J2 cells infected with PCV2, Cytochalasin D treatment could suppress PCV2 invasion, replication and release thus inhibited virus invasion [4].
References:
[1]. Sayyad, W.A., et al., The role of myosin-II in force generation of DRG filopodia and lamellipodia. Sci Rep, 2015. 5: p. 7842.
[2]. Montazeri, M., et al., Activation of Toll-like receptor 3 reduces actin polymerization and adhesion molecule expression in endometrial cells, a potential mechanism for viral-induced implantation failure. Hum Reprod, 2015.
[3]. Volkman, L.E., Baculoviruses and nucleosome management. Virology, 2015. 476c: p. 257-263.
[4]. Yan, M., L. Zhu, and Q. Yang, Infection of porcine circovirus 2 (PCV2) in intestinal porcine epithelial cell line (IPEC-J2) and interaction between PCV2 and IPEC-J2 microfilaments. Virol J, 2014. 11: p. 193.
- Pinosylvin
Catalog No.:BCN5048
CAS No.:22139-77-1
- Podocarpusflavone A
Catalog No.:BCN5047
CAS No.:22136-74-9
- Leptocarpinine
Catalog No.:BCN3730
CAS No.:221347-12-2
- Lethedioside A
Catalog No.:BCN5046
CAS No.:221289-31-2
- Lethedoside A
Catalog No.:BCN4948
CAS No.:221289-20-9
- 7,4'-Di-O-methylapigenin 5-O-xylosylglucoside
Catalog No.:BCN1484
CAS No.:221257-06-3
- 1-NM-PP1
Catalog No.:BCC4306
CAS No.:221244-14-0
- 1-Naphthyl PP1
Catalog No.:BCC3893
CAS No.:221243-82-9
- Z-Lys(Boc)-OH.DCHA
Catalog No.:BCC2591
CAS No.:2212-76-2
- Z-Lys-OH
Catalog No.:BCC2764
CAS No.:2212-75-1
- Glyoxalase I inhibitor
Catalog No.:BCC1598
CAS No.:221174-33-0
- Erysubin B
Catalog No.:BCN4947
CAS No.:221150-19-2
- Fischeria A
Catalog No.:BCN3779
CAS No.:221456-63-9
- 2-Oxokolavelool
Catalog No.:BCN4672
CAS No.:221466-41-7
- 2 beta-Hydroxykolavelool
Catalog No.:BCN4671
CAS No.:221466-42-8
- 11-Hydroxytabersonine
Catalog No.:BCN5050
CAS No.:22149-28-6
- Kaempferol 3-gentiobioside
Catalog No.:BCN3345
CAS No.:22149-35-5
- Hopane-3beta,22-diol
Catalog No.:BCN4852
CAS No.:22149-65-1
- Stigmastane-3,6-dione
Catalog No.:BCN5051
CAS No.:22149-69-5
- 1-Benzyl-1H-indazol-3-ol
Catalog No.:BCC8458
CAS No.:2215-63-6
- 2,6-Dimethoxy-1-acetonylquinol
Catalog No.:BCN5052
CAS No.:2215-96-5
- Boron tributoxide
Catalog No.:BCN8281
CAS No.:688-74-4
- L-Menthol
Catalog No.:BCN4971
CAS No.:2216-51-5
- OBAA
Catalog No.:BCC6716
CAS No.:221632-26-4
Cytochalasin D promotes pulmonary metastasis of B16 melanoma through expression of tissue factor.[Pubmed:23615686]
Oncol Rep. 2013 Jul;30(1):478-84.
Cytochalasin D (CytD) targets actin, a ubiquitous protein in eukaryotic cells. Previous studies have focused mainly on the antitumor effects of CytD. We previously found CytD to promote lung metastasis in B16 melanoma cells, which we had not anticipated, and, therefore, in the present study we investigated the possible underlying mechanisms. B16 melanoma cells were co-cultured with CytD and other agents and used to establish a lung metastatic model. In this B16 melanoma metastatic model, significantly increased lung metastasis and lung weight were found in CytD-treated mice, which was almost completely suppressed by tissue factor (TF) RNA interference expressed via lentivirus. The results of northern and western blot, and real-time RT-PCR analysis showed that the expression of TF was significantly upregulated in B16 cells treated with CytD but was significantly inhibited by TF RNA interference. In addition, upregulation and phosphorylation of mitogen-activated protein kinase p38 were also found in the metastatic lung tissues treated with CytD and in the B16 cells co-cultured with CytD and factor VIIa (FVIIa), but not in cells cultured with CytD, dimethyl sulfoxide or FVIIa alone. These results indicate that CytD stimulates the expression of TF in B16 melanoma cells, activating both coagulation-dependent and -independent pathways via binding to FVIIa, eventually promoting lung metastasis. TF interference is a potential approach to the prevention of B16 melanoma metastasis.
Staurosporine and cytochalasin D induce chondrogenesis by regulation of actin dynamics in different way.[Pubmed:22684244]
Exp Mol Med. 2012 Sep 30;44(9):521-8.
Actin cytoskeleton has been known to control and/or be associated with chondrogenesis. Staurosporine and Cytochalasin D modulate actin cytoskeleton and affect chondrogenesis. However, the underlying mechanisms for actin dynamics regulation by these agents are not known well. In the present study, we investigate the effect of staurosporine and Cytochalasin D on the actin dynamics as well as possible regulatory mechanisms of actin cytoskeleton modulation. Staurosporine and Cytochalasin D have different effects on actin stress fibers in that staurosporine dissolved actin stress fibers while Cytochalasin D disrupted them in both stress forming cells and stress fiber-formed cells. Increase in the G-/F-actin ratio either by dissolution or disruption of actin stress fiber is critical for the chondrogenic differentiation. Cytochalasin D reduced the phosphorylation of cofilin, whereas staurosporine showed little effect on cofilin phosphorylation. Either staurosporine or Cytochalasin D had little effect on the phosphorylation of myosin light chain. These results suggest that staurosporine and Cytochalasin D employ different mechanisms for the regulation of actin dynamics and provide evidence that removal of actin stress fibers is crucial for the chondrogenic differentiation.
Cytochalasin D, a tropical fungal metabolite, inhibits CT26 tumor growth and angiogenesis.[Pubmed:22305779]
Asian Pac J Trop Med. 2012 Mar;5(3):169-74.
OBJECTIVE: To investigate whether Cytochalasin D can induce antitumor activities in a tumor model. METHODS: Murine CT26 colorectal carcinoma cells were cultured in vitro and Cytochalasin D was used as a cytotoxic agent to detect its capabilities of inhibiting CT26 cell proliferation and inducing cell apoptosis by MTT and a TUNEL-based apoptosis assay. Murine CT26 tumor model was established to observe the tumor growth and survival time. Tumor tissues were used to detect the microvessel density by immunohistochemistry. In addition, alginate encapsulated tumor cell assay was used to quantify the tumor angiogenesis in vivo. RESULTS: Cytochalasin D inhibited CT26 tumor cell proliferation in time and dose dependent manner and induced significant CT26 cell apoptosis, which almost reached the level induced by the positive control nuclease. The optimum effective dose of Cytochalasin D for in vivo therapy was about 50 mg/kg. Cytochalasin D in vivo treatment significantly inhibited tumor growth and prolonged the survival times in CT26 tumor-bearing mice. The results of immunohistochemistry analysis and alginate encapsulation assay indicated that the Cytochalasin D could effectively inhibited tumor angiogenesis. CONCLUSIONS: Cytochalasin D inhibits CT26 tumor growth potentially through inhibition of cell proliferation, induction of cell apoptosis and suppression of tumor angiogenesis.
Cytochalasin D enhances the accumulation of a protease-resistant form of prion protein in ScN2a cells: involvement of PI3 kinase/Akt signalling pathway.[Pubmed:22985412]
Cell Biol Int. 2012;36(12):1223-31.
The conversion of a host-encoded PrPsen (protease-sensitive cellular prion protein) into a PrPres (protease-resistant pathogenic form) is a key process in the pathogenesis of prion diseases, but the intracellular mechanisms underlying PrPres amplification in prion-infected cells remain elusive. To assess the role of cytoskeletal proteins in the regulation of PrPres amplification, the effects of cytoskeletal disruptors on PrPres accumulation in ScN2a cells that were persistently infected with the scrapie Chandler strain have been examined. Actin microfilament disruption with Cytochalasin D enhanced PrPres accumulation in ScN2a cells. In contrast, the microtubule-disrupting agents, colchicine, nocodazole and paclitaxel, had no effect on PrPres accumulation. In addition, a PI3K (phosphoinositide 3-kinase) inhibitor, wortmannin and an Akt kinase inhibitor prevented the Cytochalasin D-induced enhancement of PrPres accumulation. Cytochalasin D-induced extension of neurite-like processes might correlate with enhanced accumulation of PrPres. The results suggest that the actin cytoskeleton and PI3K/Akt pathway are involved in the regulation of PrPres accumulation in prion-infected cells.
Cytochalasin D inhibits penetration of hamster eggs by guinea pig and human spermatozoa.[Pubmed:2777719]
J Androl. 1989 Jul-Aug;10(4):275-82.
Fertilization experiments using zona-free hamster eggs and spermatozoa from both guinea pig and human were conducted in the presence of Cytochalasin D to evaluate the possible role of actin filaments in fertilization processes. When the actin filament inhibitor, Cytochalasin D, was added to fertilization media at concentrations of 10 to 30 microM, penetration of eggs was significantly inhibited. Preincubation of the eggs with Cytochalasin D and washing prior to addition of spermatozoa had no effect on penetration as quantitated by the number of swollen heads in the egg cytoplasm. However, spermatozoa preincubated with Cytochalasin D and subsequently washed prior to egg addition showed reduced penetration of the same magnitude as when spermatozoa and eggs were coincubated with Cytochalasin D. Both the percentage of zona-free eggs showing decondensed sperm heads and the penetration indices (total decondensed spermatozoa/total eggs) were significantly affected when spermatozoa were exposed to Cytochalasin D. The DMSO vehicle used to dissolve Cytochalasin D had little effect on the number of decondensed heads. When the concentration of Cytochalasin D was increased (DMSO remaining constant) in human sperm experiments, percent penetration decreased and progressively fewer decondensed spermatozoa were recorded, indicating dose-responsiveness to Cytochalasin D. Motility parameters of human spermatozoa were not altered at any of the concentrations of Cytochalasin D tested. Neither guinea pig sperm motility nor acrosome reaction was altered significantly by Cytochalasin D or the DMSO vehicle. These experiments suggest that Cytochalasin D may be an inhibitor of some fertilization processes such as sperm penetration or sperm head decondensation.
Effects of cytochalasin D and methylamine on intracellular growth of Legionella pneumophila in amoebae and human monocyte-like cells.[Pubmed:1997428]
Infect Immun. 1991 Mar;59(3):758-63.
A cloned and axenically cultured strain of Hartmannella vermiformis was used as a model to study intracellular multiplication of Legionella pneumophila in amoebae. The growth of L. pneumophilia in both H. vermiformis and a human monocyte-like cell line (U937) was investigated with cytoskeletal and metabolic inhibitors. L. pneumophila replicated only intracellularly in these cellular models, and electron microscopy showed ultrastructural similarities in the initial phase of multiplication. Treatment of amoebae with an inhibitor of microfilament-dependent phagocytosis (Cytochalasin D, 0.5 or 1.0 micrograms/ml) did not inhibit intracellular growth of L. pneumophila; however, intracellular multiplication was inhibited by treatment of U937 monocytes with the same concentrations of Cytochalasin D. Methylamine (10 to 100 mM), an inhibitor of adsorptive pinocytosis, inhibited the replication of L. pneumophila in amoebae in a dose-dependent manner. All doses of methylamine tested (10 to 50 mM) inhibited growth of L. pneumophila in U937 monocytes. Cytochalasin D and methylamine had no effect on the multiplication of L. pneumophila in culture medium or on the viability of amoebae or U937 monocytes. Intracellular replication of L. pneumophila in H. vermiformis may be accomplished by a Cytochalasin D-independent mechanism, such as adsorptive pinocytosis. In contrast, both Cytochalasin D- and methylamine-sensitive mechanisms may be essential for the intracellular multiplication of L. pneumophila in U937 monocytes.
Inhibition of cytoplasmic streaming by cytochalasin D is superior to paraformaldehyde fixation for measuring FRET between fluorescent protein-tagged Golgi components.[Pubmed:23520174]
Cytometry A. 2013 Sep;83(9):830-8.
Protein-protein interaction at the organelle level can be analyzed by using tagged proteins and assessing Forster resonance energy transfer (FRET) between fluorescent donor and acceptor proteins. Such studies are able to uncover partners in the regulation of proteins and enzymes. However, any organelle movement is an issue for live FRET microscopy, as the observed organelle must not change position during measurement. One of the mobile organelles in plants is the Golgi apparatus following cytoplasmic streaming. It is involved in the decoration of proteins and processing of complex glycan structures for the cell wall. Understanding of these processes is still limited, but evidence is emerging that protein-protein interaction plays a key role in the function of this organelle. In the past, mobile organelles were usually immobilized with paraformaldehyde (PFA) for FRET-based interaction studies. Here, we show that the actin inhibitor Cytochalasin D (CytD) is superior to PFA for immobilization of Golgi stacks in plant cells. Two glycosyltransferases known to interact were tagged with cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP), respectively, coexpressed in Nicotiana benthamiana leaves and analyzed using confocal microscopy and spectral imaging. Fixation with PFA leads to reduced emission intensity when compared to CytD treatment. Furthermore, the calculated FRET efficiency was significantly higher with CytD than with PFA. The documented improvements are beneficial for all methods measuring FRET, where immobilization of the investigated molecules is necessary. It can be expected that FRET measurement in organelles of animal cells will also benefit from the use of inhibitors acting on the cytoskeleton.
Cytochalasin D inhibits smooth muscle contraction by directly inhibiting contractile apparatus.[Pubmed:8845566]
J Smooth Muscle Res. 1996 Apr;32(2):51-60.
We investigated the mode of relaxant effects of Cytochalasin D, a capping agent of actin filaments, on contractile responses in the rat aorta and chicken gizzard smooth muscles. Cytochalasin D inhibited the contraction induced by high K+ or noradrenaline (10 nM-1 microM) without changing cytosolic Ca2+ level ([Ca2+]i) in the rat aorta. In the absence of external Ca2+, 12-deoxyphorbol 13-isobutylate (DPB) (1 microM) induced sustained contraction without increasing in [Ca2+]i and Cytochalasin D also inhibited this contraction. In the permeabilized chicken gizzard smooth muscle, Cytochalasin D inhibited the Ca2+ (1-10 microM)-induced contraction. Cytochalasin D also inhibited the Ca(2+)-independent contraction in the muscle which had been thiophosphorylated by ATP gamma S. Cytochalasin D decreased the velocity of superprecipitation in the chicken gizzard native actomyosin (myosin B) affecting neither the level of MLC phosphorylation nor Mg(2+)-ATPase activity. These results suggest that Cytochalasin D inhibits smooth muscle contractions without any effect on the Ca(2+)-dependent MLC phosphorylation or subsequent activation of myosin ATPase activity. Based on these evidences, it is concluded that Cytochalasin D may inhibit smooth muscle contraction possibly through uncoupling of the force generation from the activated actomyosin Mg(2+)-ATPase.
Cytochalasin D stimulation of tyrosine phosphorylation and phosphotyrosine-associated kinase activity in vascular smooth muscle cells.[Pubmed:9588169]
Biochem Biophys Res Commun. 1998 Apr 28;245(3):646-50.
The actin filament-disrupting agent Cytochalasin D strikingly increased tyrosine phosphorylation of a 75 kDa protein (p75) in rabbit aortic vascular smooth muscle cells. The microtubule-disrupting agent, colchicine had no effect on p75 tyrosine phosphorylation. Cytochalasin D also stimulated p75-directed kinase activity as determined by kinase assays of anti-Tyr(P) immunoprecipitates. Cytochalasin D stimulated tyrosine phosphorylation of the F-actin-binding protein, p80/85 cortactin, but p75 was not immunologically related either to cortactin, the phosphatidylinositol 3'-kinase p85 alpha subunit, or the 80 kDa isoform of caldesmon. These results suggest that p75 may represent a Cytochalasin D-inducible kinase or kinase-associated component and provide evidence for the existence of a potentially novel kinase pathway regulated by disruption of the actin cytoskeleton.
Concentration-dependent effects of cytochalasin D on tight junctions and actin filaments in MDCK epithelial cells.[Pubmed:8006058]
J Cell Sci. 1994 Mar;107 ( Pt 3):367-75.
The effects of different concentrations of the actin-disrupting drug Cytochalasin D on tight junction permeability and distribution of actin filaments in MDCK epithelial cells were examined. Consistent with previous studies, 2 micrograms/ml Cytochalasin D caused a significant decrease in transepithelial resistance, indicative of an increase in tight junction permeability. Surprisingly, increasing concentrations of Cytochalasin D caused progressively smaller decreases in transepithelial resistance. The effects of Cytochalasin D were reversible. Light microscopic analysis utilizing rhodamine-conjugated phalloidin demonstrated two distinct populations of actin filaments in MDCK cells: an apical peripheral ring of actin, presumably associated with the zonula adherens, and larger actin bundles more basally situated. When treated with 2 micrograms/ml Cytochalasin D, both actin populations were severely disrupted and cells became flattened. Actin in the apical ring aggregated along cell boundaries, and these aggregates co-localized with similarly disrupted focal accumulations of the tight junction-associated protein ZO-1. The basal actin filament bundles also reorganized into focal aggregates. Increasing concentrations of Cytochalasin D caused gradually less perturbation of the apical actin ring, consistent with the transepithelial resistance observations. However, the basal actin bundles were disrupted at all concentrations of Cytochalasin D tested, demonstrating that the two actin populations are differentially sensitive to Cytochalasin D and that apical actin filaments are more important in the regulation of tight junction permeability. Finally, treatment of cells with Cytochalasin D inhibited the decrease in transepithelial resistance induced by the chelation of extracellular Ca2+.(ABSTRACT TRUNCATED AT 250 WORDS)
Interaction of cytochalasin D with actin filaments in the presence of ADP and ATP.[Pubmed:3944126]
J Biol Chem. 1986 Feb 15;261(5):2041-50.
Cytochalasin D strongly inhibits the faster components in the reactions of actin filament depolymerization and elongation in the presence of 10 mM Tris-Cl-, pH 7.8, 0.2 mM dithiothreitol, 1 mM MgCl2, 0.1 mM CaCl2, and 0.2 mM ATP or ADP. Assuming an exclusive and total capping of the barbed end by the drug, the kinetic parameters derived at saturation by Cytochalasin D refer to the pointed end and are 10-15-fold lower than at the barbed end. In ATP, the critical concentration increases with Cytochalasin D up to 12-fold its value when both ends are free; as a result of the lowering of the free energy of nucleation by Cytochalasin D, short oligomers of F-actin exist just above and below the critical concentration. Cytochalasin D interacts strongly with the barbed ends independently of the ADP-G-actin concentration (K = 0.5 nM-1). In contrast, the affinity of Cytochalasin D decreases cooperatively with increasing ATP-G-actin concentration. These data are equally well accounted for by two different models: either Cytochalasin D binds very poorly to ATP-capped filament ends whose proportion increases with actin concentration, or Cytochalasin D binds equally well to ATP-ends and ADP-ends and also binds to actin dimers in ATP but not in ADP. A linear actin concentration dependence of the rate of growth was found at the pointed end, consistent with the virtual absence of an ATP cap at that end.


