TopiroxostatXanthine oxidoreductase (XOR) inhibitor CAS# 577778-58-6 |
- Dorzolamide HCl
Catalog No.:BCC2311
CAS No.:130693-82-2
- Brinzolamide
Catalog No.:BCC2313
CAS No.:138890-62-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 577778-58-6 | SDF | Download SDF |
| PubChem ID | 5288320 | Appearance | Powder |
| Formula | C13H8N6 | M.Wt | 248.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | FYX-051 | ||
| Solubility | DMSO : 23.5 mg/mL (94.67 mM; Need ultrasonic and warming) | ||
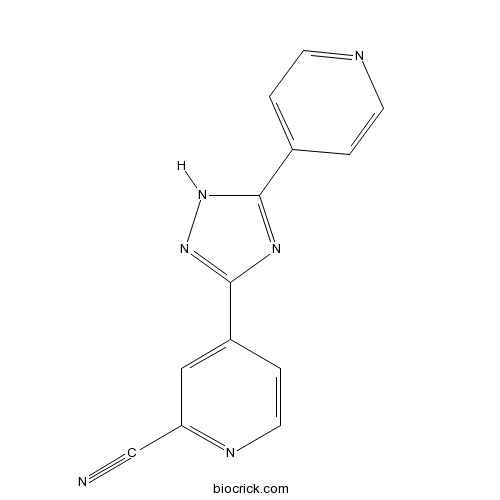
| Chemical Name | 4-(5-pyridin-4-yl-1H-1,2,4-triazol-3-yl)pyridine-2-carbonitrile | ||
| SMILES | C1=CN=CC=C1C2=NC(=NN2)C3=CC(=NC=C3)C#N | ||
| Standard InChIKey | UBVZQGOVTLIHLH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H8N6/c14-8-11-7-10(3-6-16-11)13-17-12(18-19-13)9-1-4-15-5-2-9/h1-7H,(H,17,18,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Topiroxostat(FYX-051) is a novel and potent xanthine oxidoreductase (XOR) inhibitor with IC50 value of 5.3 nM.
IC50 value: 5.3 nM [1]
Target: xanthine oxidoreductase
in vitro: Steady-state kinetics study showed that FYX-051 initially behaved as a competitive-type inhibitor with a K(i) value of 5.7 × 10(-9) M, then after a few minutes it formed a tight complex with XOR via a Mo-oxygen-carbon atom covalent linkage, as reported previously [3].
in vivo: FYX-051 exhibited a weak CYP3A4-inhibitory activity (18.6%); its Cmax and bioavailability were as high as 4.62 μg/mL (3 mg/kg) and 69.6%, respectively. Moreover, the t1/2 value of 39 was greater (19.7 h) than that of compound 2 (0.97 h) [1]. In the mechanistic study by 52-week oral treatment with topiroxostat at 3 mg/kg to F344 male rats, with and without citrate, simple and papillary transitional cell hyperplasias of the urinary bladder epithelium were observed in 5/17 in the topiroxostat-alone treatment group, along with xanthine-induced nephropathy, in contrast to neither xanthine crystals nor lesions in urinary organs by co-treatment group with citrate [2]. References: | |||||

Topiroxostat Dilution Calculator

Topiroxostat Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0284 mL | 20.1418 mL | 40.2836 mL | 80.5672 mL | 100.709 mL |
| 5 mM | 0.8057 mL | 4.0284 mL | 8.0567 mL | 16.1134 mL | 20.1418 mL |
| 10 mM | 0.4028 mL | 2.0142 mL | 4.0284 mL | 8.0567 mL | 10.0709 mL |
| 50 mM | 0.0806 mL | 0.4028 mL | 0.8057 mL | 1.6113 mL | 2.0142 mL |
| 100 mM | 0.0403 mL | 0.2014 mL | 0.4028 mL | 0.8057 mL | 1.0071 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Topiroxostat(FYX-051) is a novel and potent xanthine oxidoreductase (XOR) inhibitor with IC50 value of 5.3 nM.
- Cardionogen 1
Catalog No.:BCC6199
CAS No.:577696-37-8
- WAY 629 hydrochloride
Catalog No.:BCC7271
CAS No.:57756-44-2
- Nisoxetine hydrochloride
Catalog No.:BCC6894
CAS No.:57754-86-6
- Equisetin
Catalog No.:BCN1835
CAS No.:57749-43-6
- 4-(Ethoxymethyl)phenol
Catalog No.:BCN4753
CAS No.:57726-26-8
- ent-11alpha-Hydroxy-15-oxokaur-16-en-19-oic acid
Catalog No.:BCN7372
CAS No.:57719-81-0
- 11,15-Dihydroxy-16-kauren-19-oic acid
Catalog No.:BCN1413
CAS No.:57719-76-3
- CGP 7930
Catalog No.:BCC7096
CAS No.:57717-80-3
- Kansuinine A
Catalog No.:BCN3765
CAS No.:57701-86-7
- 2-Acetylbenzoic acid
Catalog No.:BCN5786
CAS No.:577-56-0
- Flavanomarein
Catalog No.:BCN6429
CAS No.:577-38-8
- Kansuinine B
Catalog No.:BCN3766
CAS No.:57685-46-8
- 4-(2-Hydroxy-1-methoxyethyl)-1,2-benzenediol
Catalog No.:BCN1412
CAS No.:577976-26-2
- 8-Aminoquinoline
Catalog No.:BCC8784
CAS No.:578-66-5
- Cosmosiin
Catalog No.:BCN5788
CAS No.:578-74-5
- Liquiritigenin
Catalog No.:BCN5946
CAS No.:578-86-9
- Domperidone
Catalog No.:BCC4461
CAS No.:57808-66-9
- Stevioside
Catalog No.:BCN6305
CAS No.:57817-89-7
- Idarubicin HCl
Catalog No.:BCC1194
CAS No.:57852-57-0
- Clozapine
Catalog No.:BCC5037
CAS No.:5786-21-0
- Myrianthic acid 3,23-acetonide
Catalog No.:BCN7517
CAS No.:578710-52-8
- Oligomycin A
Catalog No.:BCC2530
CAS No.:579-13-5
- Lobelanine
Catalog No.:BCN2156
CAS No.:579-21-5
- o-Anisic acid
Catalog No.:BCC9108
CAS No.:579-75-9
Clinical efficacy and safety of topiroxostat in Japanese hyperuricemic patients with or without gout: a randomized, double-blinded, controlled phase 2b study.[Pubmed:27832384]
Clin Rheumatol. 2017 Mar;36(3):649-656.
Topiroxostat, a selective xanthine oxidoreductase inhibitor, is used in Japan for the treatment of hyperuricemic patients with or without gout. In terms of the effectiveness of Topiroxostat in lowering serum urate levels, the dose-response relationship has been evaluated; however, it remains to be verified. A randomized, multi-center, double-blinded study of Topiroxostat was performed for Japanese hyperuricemic patients with or without gout. During the 16-week study, 157 Japanese hyperuricemic patients with or without gout were randomly assigned to receive a placebo, Topiroxostat at 120 or 160 mg/day, or allopurinol at 200 mg/day. The primary endpoint of this study was to determine the lowering rate of serum uric acid levels compared to those of baseline at the end of administration. A dose-response relationship (regarding decreases in the serum urate levels) was confirmed for the placebo and Topiroxostat at 120 and at 160 mg/day. Moreover, at the end of administration, the lowering rate of serum urate levels was determined to be -44.8% in the Topiroxostat 160-mg/day group. No significant difference in the incidence of adverse events was observed among all groups, including the allopurinol group. The serum urate-lowering effect of Topiroxostat was found to have a dose-response relationship in Japanese hyperuricemic patients with or without gout.
Clinical Effects of Topiroxostat on Renal and Endothelial Function in A Patient with Chronic Kidney Disease and Hyperuricemic Arteriolopathy: A Case Report.[Pubmed:28074335]
Drugs R D. 2017 Mar;17(1):97-101.
Hyperuricemia is associated with the progression of chronic kidney disease (CKD) and cardiovascular diseases. Topiroxostat, a selective xanthine oxidase inhibitor, effectively reduces serum uric acid (UA) levels and urinary albumin excretion (UAE) in CKD patients. A 50-year-old Japanese man was referred to our hospital due to albuminuria and hyperuricemia, and renal biopsy showed a typical hyperuricemic arteriolopathy. Treatment with Topiroxostat decreased serum UA levels (9.2 mg/dL at baseline to 6.4 mg/dL after 6 months), UAE (388 to 88 mg/g.cr), and urinary level of liver-type fatty acid-binding protein (28.8 to 19.8 microg/g.cr). Interestingly, Topiroxostat treatment was associated with a trend towards improved flow-mediated dilation (5.4 to 5.8%). These results suggested that Topiroxostat in CKD patients with hyperuricemia is potentially effective, not only for ameliorating renal damages but also for improving endothelial function beyond its UA-lowering action.
Randomized control trial for the assessment of the anti-albuminuric effects of topiroxostat in hyperuricemic patients with diabetic nephropathy (the ETUDE study).[Pubmed:27303100]
Nagoya J Med Sci. 2016 May;78(2):135-42.
Proteinuria is an established risk factor for diabetic nephropathy. Recent studies indicate that some xanthine oxidase inhibitors have a renoprotective effect. The aim of this study was to assess whether Topiroxostat reduces albuminuria in hyperuricemic patients with diabetic nephropathy and overt proteinuria. The ETUDE study is an ongoing 24-week, multicenter, open-label, randomized (1:1), parallel group study involving hyperuricemic patients with diabetic nephropathy (estimated glomerular filtration rate [eGFR] >/= 20 mL/min/1.73 m(2)) and overt proteinuria (0.3 Topiroxostat 160 mg daily) or low dose (Topiroxostat 40 mg daily) on top of standard of care. The primary endpoint is the change in albuminuria indicated by urine albumin-to-creatinine ratio after 24 treated weeks relative to the baseline values. This trial was registered at the Japanese University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR: UMIN 000015403). The background, rationale, and study design of this trial are presented here. Seventy-six patients from four registered facilities have already been enrolled and received at least one dose of Topiroxostat. This trial will end in 2017. The ETUDE trial is the first randomized controlled study of Topiroxostat in hyperuricemic patients with diabetic nephropathy and overt proteinuria. We will clarify the pleiotropic function of Topiroxostat including an anti-albumiuric effect as well as its effects on safely decreasing serum uric acid levels.


