ent-11alpha-Hydroxy-15-oxokaur-16-en-19-oic acidCAS# 57719-81-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 57719-81-0 | SDF | Download SDF |
| PubChem ID | 21593620 | Appearance | Powder |
| Formula | C20H28O4 | M.Wt | 332.44 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
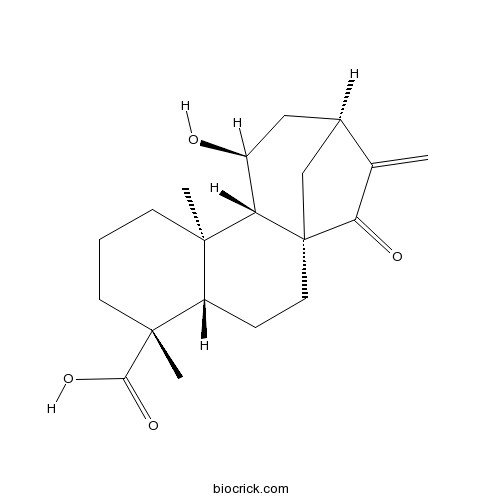
| Chemical Name | (1R,4S,5R,9R,10S,11S,13S)-11-hydroxy-5,9-dimethyl-14-methylidene-15-oxotetracyclo[11.2.1.01,10.04,9]hexadecane-5-carboxylic acid | ||
| SMILES | CC12CCCC(C1CCC34C2C(CC(C3)C(=C)C4=O)O)(C)C(=O)O | ||
| Standard InChIKey | NSFLYGNWNATSHL-UKFLMKJWSA-N | ||
| Standard InChI | InChI=1S/C20H28O4/c1-11-12-9-13(21)15-18(2)6-4-7-19(3,17(23)24)14(18)5-8-20(15,10-12)16(11)22/h12-15,21H,1,4-10H2,2-3H3,(H,23,24)/t12-,13+,14+,15+,18-,19-,20-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Ent-11alpha-Hydroxy-15-oxokaur-16-en-19-oic acid inhibits hepatocellular carcinoma in vitro and in vivo via stabilizing IkBα. 2. Ent-11alpha-Hydroxy-15-oxokaur-16-en-19-oic acid induces apoptosis of human malignant cancer cells. |
| Targets | p65 | NF-kB | Bcl-2/Bax | ERK | p53 | p38MAPK | Caspase | JNK |

ent-11alpha-Hydroxy-15-oxokaur-16-en-19-oic acid Dilution Calculator

ent-11alpha-Hydroxy-15-oxokaur-16-en-19-oic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0081 mL | 15.0403 mL | 30.0806 mL | 60.1612 mL | 75.2015 mL |
| 5 mM | 0.6016 mL | 3.0081 mL | 6.0161 mL | 12.0322 mL | 15.0403 mL |
| 10 mM | 0.3008 mL | 1.504 mL | 3.0081 mL | 6.0161 mL | 7.5202 mL |
| 50 mM | 0.0602 mL | 0.3008 mL | 0.6016 mL | 1.2032 mL | 1.504 mL |
| 100 mM | 0.0301 mL | 0.1504 mL | 0.3008 mL | 0.6016 mL | 0.752 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 11,15-Dihydroxy-16-kauren-19-oic acid
Catalog No.:BCN1413
CAS No.:57719-76-3
- CGP 7930
Catalog No.:BCC7096
CAS No.:57717-80-3
- Kansuinine A
Catalog No.:BCN3765
CAS No.:57701-86-7
- 2-Acetylbenzoic acid
Catalog No.:BCN5786
CAS No.:577-56-0
- Flavanomarein
Catalog No.:BCN6429
CAS No.:577-38-8
- Kansuinine B
Catalog No.:BCN3766
CAS No.:57685-46-8
- Palmitic acid-1-13C
Catalog No.:BCC8229
CAS No.:57677-53-9
- Baccatin VI
Catalog No.:BCN7229
CAS No.:57672-79-4
- 1-Dehydroxybaccatin IV
Catalog No.:BCN7211
CAS No.:57672-78-3
- Baccatin IV
Catalog No.:BCN5785
CAS No.:57672-77-2
- Fenobam
Catalog No.:BCC7345
CAS No.:57653-26-6
- Piperenone
Catalog No.:BCN6578
CAS No.:57625-31-7
- 4-(Ethoxymethyl)phenol
Catalog No.:BCN4753
CAS No.:57726-26-8
- Equisetin
Catalog No.:BCN1835
CAS No.:57749-43-6
- Nisoxetine hydrochloride
Catalog No.:BCC6894
CAS No.:57754-86-6
- WAY 629 hydrochloride
Catalog No.:BCC7271
CAS No.:57756-44-2
- Cardionogen 1
Catalog No.:BCC6199
CAS No.:577696-37-8
- Topiroxostat
Catalog No.:BCC4202
CAS No.:577778-58-6
- 4-(2-Hydroxy-1-methoxyethyl)-1,2-benzenediol
Catalog No.:BCN1412
CAS No.:577976-26-2
- 8-Aminoquinoline
Catalog No.:BCC8784
CAS No.:578-66-5
- Cosmosiin
Catalog No.:BCN5788
CAS No.:578-74-5
- Liquiritigenin
Catalog No.:BCN5946
CAS No.:578-86-9
- Domperidone
Catalog No.:BCC4461
CAS No.:57808-66-9
- Stevioside
Catalog No.:BCN6305
CAS No.:57817-89-7
Ent-11alpha-hydroxy-15-oxo-kaur-16-en-19-oic-acid induces apoptosis of human malignant cancer cells.[Pubmed:23140284]
Curr Drug Targets. 2012 Dec;13(14):1730-7.
Ent-11alpha-hydroxy-15-oxo-kaur-16-en-19-oic-acid (5F) is a chemical compound isolated from Pteris semipinnata L (PsL), a Chinese traditional herb. 5F has been known to exert antitumor activity in several kinds of human malignant cancer cells by leading cancer cell to apoptosis. 5F translocated Bax into the mitochondria, down-regulated Bcl-2, activated caspase-9 and caspase-3, released cytochrome c into the cytosol and translocated AIF from the mitochondria to the nucleus. The presentation of a wild-type p53 in the cancer cells facilitated cancer cells sensitive to the 5F treatment. 5F induces apoptosis of cancer cells by inhibiting NF-kappaB activation/induction, which leading to the decrease of Bcl-2 but the increase of Bax and Bak. MAPK kinases and Akt are also involved in process of 5F inducing cancer cell apoptosis. In lung cancer, 5F activated ERK1/2 and the inhibition of ERK1/2 suppressed 5F-mediated changes in apoptotic molecules. 5F activated Akt and suggested that Akt activation was anti-apoptotic rather than pro-apoptotic. However, in anaplastic thyroid carcinoma, JNK activation was related to cell death induced by 5F. ERK and p38 were also activated but as survival signals in response to 5F treatment to counteract the induction of cell death. Collectively, 5F is effective against several malignant cancers both in vivo and in vitro with minimal side effects. It induces apoptosis through the mitochondrialmediated pathway, in which regulation of Bcl-2 family proteins expression, the activation of MAPK and inactivation of NF-kappaB are critical. The good ability of 5F to inhibit cancer cells makes it in line with the successful development of other anti-tumor agents.
Ent-11alpha-hydroxy-15-oxo-kaur-16-en-19-oic-acid inhibits hepatocellular carcinoma in vitro and in vivo via stabilizing IkBalpha.[Pubmed:22227815]
Invest New Drugs. 2012 Dec;30(6):2210-8.
Ent-11-hydroxy-15-oxo-kaur-16-en-19-oic-acid (5F) isolated from Pteris Semipinnata L is known to inhibit certain tumor cells in vitro. The information on the in vivo effect of 5F is limited and its effect on hepatocellular carcinoma (HCC) is unknown. In this study, the anti-tumor effect of 5F was investigated in a diethylnitrosamine (DEN)-induced mouse HCC model. In addition to therapeutic effect, the potential side effect was monitored. A panel of cultured HCC cells was used to confirm the in vivo data and explore the responsible molecular pathway. The result showed that 5F significantly inhibited the DEN-induced HCC tumors by reducing the number of tumor foci and the volume of tumors. Furthermore, 5F induced the death of cultured HCC cells in dose- and time-dependent manners. The cell death was confirmed to be apoptotic by in vivo and in vitro TUNEL assays. 5F inhibited NF-kB by stabilizing its inhibitor IkBalpha, reducing the nuclear p65 and inhibiting NF-kB activity. Subsequently it affected the NF-kB downstream molecules with a decrease in anti-apoptotic Bcl-2 and increase in pro-apoptotic Bax and Bak. During the whole period of the experiment, mice receiving 5F appeared to be healthy, though they suffered from a mild degree of hair loss. 5F did not damage liver and renal functions. In conclusion, 5F is effective against HCC with minimal side effects. It induces apoptosis in HCC cells via inhibiting NF-kB, leading to the decrease of Bcl-2 but the increase of Bax and Bak.


