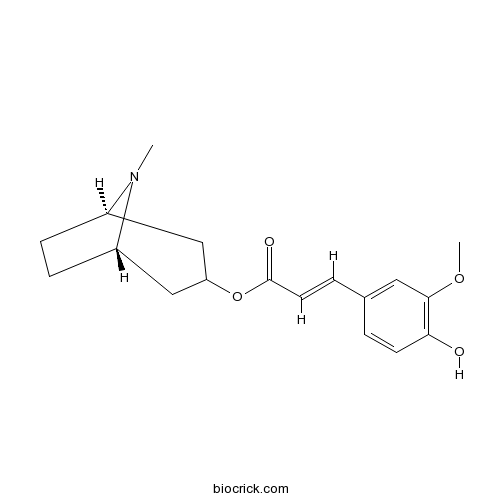
Tropanyl 3-hydroxy-4-methoxycinnamateCAS# 86702-58-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 86702-58-1 | SDF | Download SDF |
| PubChem ID | 6440788 | Appearance | Powder |
| Formula | C18H23NO4 | M.Wt | 317.38 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1S,5R)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl] (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate | ||
| SMILES | CN1C2CCC1CC(C2)OC(=O)C=CC3=CC(=C(C=C3)O)OC | ||
| Standard InChIKey | RSPBFOSKTPFBGJ-LYEGFCJJSA-N | ||
| Standard InChI | InChI=1S/C18H23NO4/c1-19-13-5-6-14(19)11-15(10-13)23-18(21)8-4-12-3-7-16(20)17(9-12)22-2/h3-4,7-9,13-15,20H,5-6,10-11H2,1-2H3/b8-4+/t13-,14+,15? | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Tropanyl 3-hydroxy-4-methoxycinnamate Dilution Calculator

Tropanyl 3-hydroxy-4-methoxycinnamate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1508 mL | 15.754 mL | 31.508 mL | 63.0159 mL | 78.7699 mL |
| 5 mM | 0.6302 mL | 3.1508 mL | 6.3016 mL | 12.6032 mL | 15.754 mL |
| 10 mM | 0.3151 mL | 1.5754 mL | 3.1508 mL | 6.3016 mL | 7.877 mL |
| 50 mM | 0.063 mL | 0.3151 mL | 0.6302 mL | 1.2603 mL | 1.5754 mL |
| 100 mM | 0.0315 mL | 0.1575 mL | 0.3151 mL | 0.6302 mL | 0.7877 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Magnoshinin
Catalog No.:BCC8205
CAS No.:86702-02-5
- ROCK inhibitor
Catalog No.:BCC1905
CAS No.:867017-68-3
- (S)-Methylisothiourea sulfate
Catalog No.:BCC6791
CAS No.:867-44-7
- (R)-(+)-2-Amino-3-methyl-1,1-diphenyl-1-butanol
Catalog No.:BCC8394
CAS No.:86695-06-9
- ARL 17477 dihydrochloride
Catalog No.:BCC7647
CAS No.:866914-87-6
- BINA
Catalog No.:BCC7849
CAS No.:866823-73-6
- (1S)-4,5-Dimethoxy-1-[(methylamino)methyl]benzocyclobutane hydrochloride
Catalog No.:BCC8383
CAS No.:866783-13-3
- Yuexiandajisu E
Catalog No.:BCN3775
CAS No.:866556-16-3
- Yuexiandajisu D
Catalog No.:BCN3774
CAS No.:866556-15-2
- [Ala2,8,9,11,19,22,24,25,27,28]-VIP
Catalog No.:BCC5973
CAS No.:866552-34-3
- 7-Hydroxy-3-prenylcoumarin
Catalog No.:BCN4415
CAS No.:86654-26-4
- Dorsomorphin
Catalog No.:BCC5131
CAS No.:866405-64-3
- Colivelin
Catalog No.:BCC7821
CAS No.:867021-83-8
- Linsitinib
Catalog No.:BCC3697
CAS No.:867160-71-2
- TRC 051384
Catalog No.:BCC7968
CAS No.:867164-40-7
- TG 100572 Hydrochloride
Catalog No.:BCC1995
CAS No.:867331-64-4
- TG 100801
Catalog No.:BCC1996
CAS No.:867331-82-6
- TG 100572
Catalog No.:BCC1994
CAS No.:867334-05-2
- Astrasieversianin VII
Catalog No.:BCN2788
CAS No.:86764-11-6
- CRF (human, rat)
Catalog No.:BCC5710
CAS No.:86784-80-7
- H-Cys-OEt.HCl
Catalog No.:BCC2904
CAS No.:868-59-7
- Carasiphenol C
Catalog No.:BCN8251
CAS No.:868168-04-1
- SID 7969543
Catalog No.:BCC6026
CAS No.:868224-64-0
- Pam2CSK4
Catalog No.:BCC6247
CAS No.:868247-72-7
A novel spirocyclic tropanyl-Delta(2)-isoxazoline derivative enhances citalopram and paroxetine binding to serotonin transporters as well as serotonin uptake.[Pubmed:23022052]
Bioorg Med Chem. 2012 Nov 1;20(21):6344-55.
A group of spirocyclic tropanyl-Delta(2)-isoxazolines was synthesized exploiting the 1,3-dipolar cycloaddition of nitrile oxides to olefins. Their interaction with the dopamine and serotonin transporters (DAT and SERT, respectively) was evaluated through binding experiments. The majority of the compounds had no inhibitory effects (IC(50) >> 10 muM), while some had an IC(50) value in the range 5-10 muM (8a-c, 10b and 11c on DAT, 12b on SERT). Unexpectedly, one of the tertiary amines under investigation, that is 3'-methoxy-8-methyl-spiro{8-azabicyclo[3.2.1]octane-3,5'(4'H)-isoxazole 7a, was able to enhance at a concentration of 10 muM both [(3)H]citalopram and [(3)H]paroxetine binding to SERT in rat brain homogenate (up to 25%, due to an increase of B(max)) and [(3)H]serotonin uptake (up to 30%) in cortical synaptosomes. This peculiar pharmacological profile of 7a suggests it binds to an allosteric site on SERT, and positions derivative 7a as a very useful tool to investigate SERT machinery.
Further structure-activity relationships in the series of tropanyl esters endowed with potent antinociceptive activity.[Pubmed:10230057]
Farmaco. 1998 Dec 30;53(12):764-72.
Several analogs of the alpha-tropanyl esters of 2-(4-chlorophenoxy)butyric acid (SM21) and 2-phenylthiobutyric acid (SM32), endowed with potent antinociceptive and cognition enhancing activity, were synthesized, aimed at obtaining more potent and safe drug candidates. Variation of the acyl moiety (4-11), as well as the conformational restriction of atropine to give the alpha-tropanyl ester of 2,3-dihydrobenzofurane-3-carboxylic acid (18), practically abolished activity. In the case of 18, the antimuscarinic activity was also severely affected by the conformation restrain. On the contrary, conformational restriction of phenoxybutyric and phenylthiobutyric acid derivatives to give the alpha-tropanyl ester of 2,3-dihydro-benzofurane-2-carboxylic acid and 2,3-dihydro-benzothiophene-2-carboxylic acid (12-17), afforded potent analgesic drugs that unfortunately were too toxic to be reliable drug candidates. A series of related esters of benzofurane-3-carboxylic acid (20-27) and benzothiophene-3-carboxylic acid (28) were also studied and found to be potent but toxic analgesics.
GRIND-derived pharmacophore model for a series of alpha-tropanyl derivative ligands of the sigma-2 receptor.[Pubmed:15595462]
J Comput Aided Mol Des. 2004 May;18(5):361-74.
A pharmacophore model for the sigma-2 receptor was derived using GRIND (GRid INdependent Descriptors) descriptors arising from a 3D-level procedure whose main prerogative is that it does not require ligand alignment. PLS models for sigma-2 affinity (sigma-2 model: r2=0.83, q2=0.63) and sigma-1/sigma-2 selectivity (r2=0.72, q2=0.46) were derived using a series of alpha-tropanyl derivatives. The models provide pictures of the virtual receptor site (VRS) significant enough to attain a qualitative pharmacophoric representation of the sigma receptor. They give the internal geometrical relationships within two hydrophobic areas (hydrophobic-1 and -2) and a H-bond donor receptor region with which ligands establish non-covalent bonds.
Antinociceptive profile of 3-alpha-tropanyl 2-(4-Cl-phenoxy)butyrate (SM-21) [corrected]: a novel analgesic with a presynaptic cholinergic mechanism of action.[Pubmed:9223584]
J Pharmacol Exp Ther. 1997 Jul;282(1):430-9.
The antinociceptive effect of (+/-)-3-alpha-tropanyl 2-(4-Cl-phenoxy)butyrate [corrected] (SM-21) (10-40 mg kg(-1) s.c., 10-30 mg kg(-1) i.p., 20-60 mg kg(-1) p.o., 3-20 mg kg(-1) i.v. and 5-20 microg per mouse i.c.v.) was examined in rodents and guinea pigs by using the hot-plate, abdominal constriction, tail-flick and paw-pressure tests. The antinociception produced by (+/-)-SM-21 was prevented by atropine, pirenzepine and hemicholinium-3 but not by quinpirole, R-(alpha)-methylhistamine, [1-[2(methylsufonyl)amino]ethyl]-4-piperidinyl]methyl-5-floro++ +-2-methoxy-1H-indole-3-carboxylate hydrochloride, N6-cyclopentyladenosine, 1-(2-methoxyphenyl)-4-[4-(2-phthalimido)butyl]piperazine hydrobromide, naloxone, 3-aminopropyl-diethoxy-methyl-phosphinic acid or reserpine. On the basis of the above data, it can be postulated that (+/-)-SM-21 exerted an antinociceptive effect mediated by a central potentiation of cholinergic transmission. Affinity profiles of (+/-)-SM-21 for muscarinic receptor subtypes, determined by functional studies (rabbit vas deferens for M1, guinea pig atrium for M2, guinea pig ileum for M3 and immature guinea pig uterus for putative M4) have shown a selectivity ratio M2/M1 of 4.6 that, although very low, might be responsible for the antinociception induced by (+/-)-SM-21 through an increase in ACh extracellular levels. In the antinociceptive dose range, (+/-)-SM-21 did not impair mouse performance evaluated by the rota-rod and hole-board tests.


