ARL 17477 dihydrochlorideSelective nNOS inhibitor CAS# 866914-87-6 |
- Nω-Propyl-L-arginine hydrochloride
Catalog No.:BCC6965
CAS No.:137361-05-8
- 7-NINA
Catalog No.:BCC5674
CAS No.:161467-34-1
- L-Canavanine sulfate
Catalog No.:BCC6746
CAS No.:2219-31-0
- 7-Nitroindazole
Catalog No.:BCC6713
CAS No.:2942-42-9
- L-NMMA acetate
Catalog No.:BCC6788
CAS No.:53308-83-1
- 3-Bromo-7-nitroindazole
Catalog No.:BCC6770
CAS No.:74209-34-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 866914-87-6 | SDF | Download SDF |
| PubChem ID | 9824646 | Appearance | Powder |
| Formula | C20H22Cl3N3S | M.Wt | 442.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in water and to 100 mM in DMSO | ||
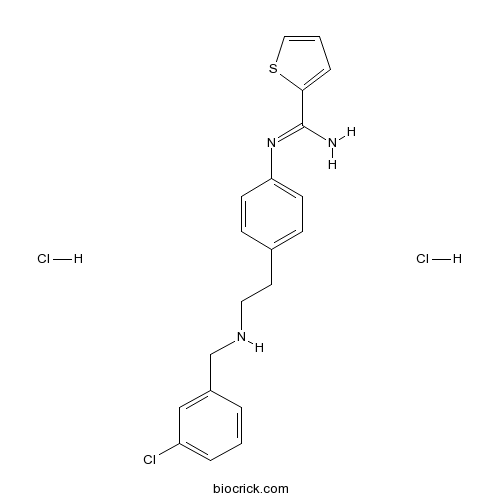
| Chemical Name | N'-[4-[2-[(3-chlorophenyl)methylamino]ethyl]phenyl]thiophene-2-carboximidamide;dihydrochloride | ||
| SMILES | C1=CC(=CC(=C1)Cl)CNCCC2=CC=C(C=C2)N=C(C3=CC=CS3)N.Cl.Cl | ||
| Standard InChIKey | SOXBYIKSUDXMIE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H20ClN3S.2ClH/c21-17-4-1-3-16(13-17)14-23-11-10-15-6-8-18(9-7-15)24-20(22)19-5-2-12-25-19;;/h1-9,12-13,23H,10-11,14H2,(H2,22,24);2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective neuronal nitrogen oxide synthase (nNOS) inhibitor (IC50 values are 1 and 17 μM for nNOS and endothelial NOS respectively). Reduces ischemic cell damage after middle cerebral artery (MCA) occlusion in rats. Displays a synergistic neuroprotective effect when combined with either an NMDA or AMPA receptor antagonist. |

ARL 17477 dihydrochloride Dilution Calculator

ARL 17477 dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2582 mL | 11.291 mL | 22.582 mL | 45.1641 mL | 56.4551 mL |
| 5 mM | 0.4516 mL | 2.2582 mL | 4.5164 mL | 9.0328 mL | 11.291 mL |
| 10 mM | 0.2258 mL | 1.1291 mL | 2.2582 mL | 4.5164 mL | 5.6455 mL |
| 50 mM | 0.0452 mL | 0.2258 mL | 0.4516 mL | 0.9033 mL | 1.1291 mL |
| 100 mM | 0.0226 mL | 0.1129 mL | 0.2258 mL | 0.4516 mL | 0.5646 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ARL 17477 dihydrochloride is a selective and potent neuronal nitrogen oxide synthase (nNOS) inhibitor with IC50 values of 1 and 17μM for nNOS and endothelial NOS, respectively [1].
Neuronal NOS (nNOS) plays an important role in the development of ischaemic brain necrosis while endothelial NOS (eNOS) protects brain tissue through increasing ischaemic regional cerebral blood flow [1].
In rats after transient MCA occlusion, ARL 17477 (1mg/kg, 3mg/kg and 10mg/kg) reduced ischemic infarct volume by 53%, 23% and 6.5% respectively in a dose-dependent way. ARL 17477 (10 mg/kg) significantly reduced regional cerebral blood flow (rCBF) by 27±5.3% and 24±14.08%. Also, it reduced cortical NOS activity by 86±14.9% and 91±8.9% at 10 min or 3 h, respectively [2]. In the global cerebral ischaemia gerbil model, the combination of MK-801 with ARL 17477 provided 44% greater protection than the total protection or either alone. Likewise, the combination of LY293558 with ARL 17477 provided 35% greater protection than total protection of either compound alone [3].
References:
[1]. O'Neill MJ, Murray TK, McCarty DR, et al. ARL 17477, a selective nitric oxide synthase inhibitor, with neuroprotective effects in animal models of global and focal cerebral ischaemia. Brain Res, 2000, 871(2): 234-244.
[2]. Zhang ZG, Reif D, Macdonald J, et al. ARL 17477, a potent and selective neuronal NOS inhibitor decreases infarct volume after transient middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab, 1996, 16(4): 599-604.
[3]. Hicks CA, Ward MA, Swettenham JB, et al. Synergistic neuroprotective effects by combining an NMDA or AMPA receptor antagonist with nitric oxide synthase inhibitors in global cerebral ischaemia. Eur J Pharmacol, 1999, 381(2-3): 113-119.
- BINA
Catalog No.:BCC7849
CAS No.:866823-73-6
- (1S)-4,5-Dimethoxy-1-[(methylamino)methyl]benzocyclobutane hydrochloride
Catalog No.:BCC8383
CAS No.:866783-13-3
- Yuexiandajisu E
Catalog No.:BCN3775
CAS No.:866556-16-3
- Yuexiandajisu D
Catalog No.:BCN3774
CAS No.:866556-15-2
- [Ala2,8,9,11,19,22,24,25,27,28]-VIP
Catalog No.:BCC5973
CAS No.:866552-34-3
- 7-Hydroxy-3-prenylcoumarin
Catalog No.:BCN4415
CAS No.:86654-26-4
- Dorsomorphin
Catalog No.:BCC5131
CAS No.:866405-64-3
- DPPI 1c hydrochloride
Catalog No.:BCC2363
CAS No.:866396-34-1
- 10-Aminocamptothecin
Catalog No.:BCC8111
CAS No.:86639-63-6
- 7-Ethyl-10-Hydroxycamptothecin
Catalog No.:BCN2479
CAS No.:86639-52-3
- Xanthiside
Catalog No.:BCN2545
CAS No.:866366-86-1
- 3,4,5-Tricaffeoylquinic acid
Catalog No.:BCN2384
CAS No.:86632-03-3
- (R)-(+)-2-Amino-3-methyl-1,1-diphenyl-1-butanol
Catalog No.:BCC8394
CAS No.:86695-06-9
- (S)-Methylisothiourea sulfate
Catalog No.:BCC6791
CAS No.:867-44-7
- ROCK inhibitor
Catalog No.:BCC1905
CAS No.:867017-68-3
- Magnoshinin
Catalog No.:BCC8205
CAS No.:86702-02-5
- Tropanyl 3-hydroxy-4-methoxycinnamate
Catalog No.:BCN1325
CAS No.:86702-58-1
- Colivelin
Catalog No.:BCC7821
CAS No.:867021-83-8
- Linsitinib
Catalog No.:BCC3697
CAS No.:867160-71-2
- TRC 051384
Catalog No.:BCC7968
CAS No.:867164-40-7
- TG 100572 Hydrochloride
Catalog No.:BCC1995
CAS No.:867331-64-4
- TG 100801
Catalog No.:BCC1996
CAS No.:867331-82-6
- TG 100572
Catalog No.:BCC1994
CAS No.:867334-05-2
- Astrasieversianin VII
Catalog No.:BCN2788
CAS No.:86764-11-6
ARL 17477, a selective nitric oxide synthase inhibitor, with neuroprotective effects in animal models of global and focal cerebral ischaemia.[Pubmed:10899290]
Brain Res. 2000 Jul 21;871(2):234-44.
In the present studies, we have evaluated the effects of N-[4-(2- inverted question mark[(3-Chlorophenyl)methyl]amino inverted question markethyl)phenyl]-2-thiophenecarbo ximidamide dihydrochloride (ARL 17477) on recombinant human neuronal NOS (nNOS) and endothelial NOS (eNOS). We then carried out pharmacokinetic studies and measured cortical nitric oxide synthase (NOS) inhibition to determine that the compound crossed the blood brain barrier. Finally, the compound was evaluated in a model of global ischaemia in the gerbil and two models of transient focal ischaemia in the rat. The IC(50) values for ARL 17477 on human recombinant human nNOS and eNOS were 1 and 17 microM, respectively. ARL 17477 (50 mg/kg i.p.) produced a significant reduction in the ischaemia-induced hippocampal damage following global ischaemia when administered immediately post-occlusion, but failed to protect when administration was delayed until 30 min post-occlusion. In the endothelin-1 model of focal ischaemia, ARL 17477 (1 mg/kg i.v.) significantly attenuated the infarct volume when administered at either 0, 1 or 2 h post-endothelin-1 (P<0.05). In the intraluminal suture model, ARL 17477 at both 1 and 3 mg/kg i.v. failed to reduce the infarct volume measured at 1, 3 or 7 days post-occlusion. These results demonstrate that ARL 17477 protects against global ischaemia in gerbils and provides some reduction in infarct volume following transient middle cerebral artery occlusion in rats, indicating that nNOS inhibition may be a useful treatment of ischaemic conditions.
Synergistic neuroprotective effects by combining an NMDA or AMPA receptor antagonist with nitric oxide synthase inhibitors in global cerebral ischaemia.[Pubmed:10554878]
Eur J Pharmacol. 1999 Sep 24;381(2-3):113-9.
We have investigated the neuroprotective effects of combining an NMDA or AMPA receptor antagonist with a nitric oxide synthase (NOS) inhibitor in the gerbil model of global cerebral ischaemia. Ischaemia was induced by occlusion of the common carotid arteries for 5 min. (5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,1 0-imine (MK-801, 2.5 mg/kg i.p.) or (3S,4aR,6R,8aR)-6-[2-(1(2)H-tetrazole-5-yl)]decahydroisoq uinoline-3-carboxylic acid (LY293558, 20 mg/kg i.p.) and 7-nitroindazole (25 mg/kg i.p.) or N-[4-(2-[[(3-chlorophenyl)methyl]amino]ethyl) phenyl]-2-thiophenecarboximidamide dihydrochloride (ARL17477, 25 mg/kg i.p.) were administered alone or in combination (i.e., MK-801 with 7-nitroindazole or ARL17477 or LY293558 with 7-nitroindazole or ARL17477). In the present studies, both MK-801 and LY293558 provided significant degree of neuroprotection, while 7-nitroindazole and ARL17477 also provided some neuroprotection, which failed to reach significance in every case. However, the combination of MK-801 with 7-nitroindazole or ARL17477 provided 21% or 44% greater protection than the total protection or either alone. Likewise, the combination of LY293558 with 7-nitroindazole or ARL17477 provided 14.5% and 35% greater protection than total protection of either compound alone. These results indicate that several pathways contribute to ischaemic cell death and combining excitatory amino antagonists and NOS inhibitors provides greater protection than either alone. Therefore, combination therapy should be considered as an approach for treating ischaemic conditions.


