UBP 302CAS# 745055-91-8 |
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 745055-91-8 | SDF | Download SDF |
| PubChem ID | 6420161 | Appearance | Powder |
| Formula | C15H15N3O6 | M.Wt | 333.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in 1eq. NaOH and to 20 mM in DMSO with gentle warming | ||
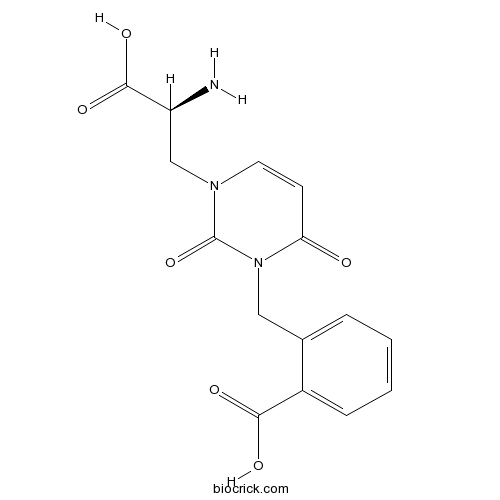
| Chemical Name | 2-[[3-[(2S)-2-amino-2-carboxyethyl]-2,6-dioxopyrimidin-1-yl]methyl]benzoic acid | ||
| SMILES | C1=CC=C(C(=C1)CN2C(=O)C=CN(C2=O)CC(C(=O)O)N)C(=O)O | ||
| Standard InChIKey | UUIYULWYHDSXHL-NSHDSACASA-N | ||
| Standard InChI | InChI=1S/C15H15N3O6/c16-11(14(22)23)8-17-6-5-12(19)18(15(17)24)7-9-3-1-2-4-10(9)13(20)21/h1-6,11H,7-8,16H2,(H,20,21)(H,22,23)/t11-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective GLUK5 (GluR5)-subunit containing kainate receptor antagonist (apparent KD = 402 nM); active enantiomer of UBP 296. Displays ~ 260-fold selectivity over AMPA receptors, ~ 90-fold selectivity over recombinant human GLUK6- and GLUK2-containing kainate receptors and has little or no action at NMDA or group I mGlu receptors. Selectively blocks kainate receptor-mediated LTP induction in rat hippocampal mossy fibers. Also protects against soman-induced seizures and neuronal degeneration for up to 30 days when administered one hour after soman exposure. |

UBP 302 Dilution Calculator

UBP 302 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0003 mL | 15.0015 mL | 30.003 mL | 60.006 mL | 75.0075 mL |
| 5 mM | 0.6001 mL | 3.0003 mL | 6.0006 mL | 12.0012 mL | 15.0015 mL |
| 10 mM | 0.3 mL | 1.5002 mL | 3.0003 mL | 6.0006 mL | 7.5008 mL |
| 50 mM | 0.06 mL | 0.3 mL | 0.6001 mL | 1.2001 mL | 1.5002 mL |
| 100 mM | 0.03 mL | 0.15 mL | 0.3 mL | 0.6001 mL | 0.7501 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- UBP 296
Catalog No.:BCC7255
CAS No.:745055-86-1
- Ivachtin
Catalog No.:BCC2357
CAS No.:745046-84-8
- MMAF
Catalog No.:BCC5144
CAS No.:745017-94-1
- Alprostadil
Catalog No.:BCC4963
CAS No.:745-65-3
- Casegravol
Catalog No.:BCN4591
CAS No.:74474-76-3
- Potassium Chloride
Catalog No.:BCC7581
CAS No.:7447-40-7
- Sauvagine
Catalog No.:BCC5792
CAS No.:74434-59-6
- Symlandine
Catalog No.:BCN1974
CAS No.:74410-74-5
- PAF (C16)
Catalog No.:BCC7522
CAS No.:74389-68-7
- Leuprolide Acetate
Catalog No.:BCC1701
CAS No.:74381-53-6
- 8-Geranyloxypsoralen
Catalog No.:BCN4296
CAS No.:7437-55-0
- 4-hydroxyephedrine hydrochloride
Catalog No.:BCC8103
CAS No.:7437-54-9
- Cristacarpin
Catalog No.:BCN3935
CAS No.:74515-47-2
- Isomedicarpin
Catalog No.:BCN4297
CAS No.:74560-05-7
- D-64131
Catalog No.:BCC1510
CAS No.:74588-78-6
- Rubiadin 1-methyl ether
Catalog No.:BCN4298
CAS No.:7460-43-7
- Tetrahydrocoptisine
Catalog No.:BCN2558
CAS No.:7461-02-1
- Tylosin tartrate
Catalog No.:BCC4875
CAS No.:74610-55-2
- ent-3Beta-Angeloyloxykaur-16-en-19-oic acid
Catalog No.:BCN1365
CAS No.:74635-61-3
- 11-Hydroxydrim-7-en-6-one
Catalog No.:BCN7770
CAS No.:74635-87-3
- Liquiritin Apioside
Catalog No.:BCC8334
CAS No.:74639-14-8
- 1,3,9-Trimethyluric acid
Catalog No.:BCN7393
CAS No.:7464-93-9
- Nuclear yellow
Catalog No.:BCC1810
CAS No.:74681-68-8
- 3-Methoxy-4,5-methylenedioxycinnamaldehyde
Catalog No.:BCN1364
CAS No.:74683-19-5
Evaluation of acetylcholine, seizure activity and neuropathology following high-dose nerve agent exposure and delayed neuroprotective treatment drugs in freely moving rats.[Pubmed:27329284]
Toxicol Mech Methods. 2016 Jun;26(5):378-88.
Organophosphorus nerve agents such as soman (GD) inhibit acetylcholinesterase, producing an excess of acetylcholine (ACh), which results in respiratory distress, convulsions and status epilepticus that leads to neuropathology. Several drugs (topiramate, clobazam, pregnanolone, allopregnanolone, UBP 302, cyclopentyladenosine [CPA], ketamine, midazolam and scopolamine) have been identified as potential neuroprotectants that may terminate seizures and reduce brain damage. To systematically evaluate their efficacy, this study employed in vivo striatal microdialysis and liquid chromatography to respectively collect and analyze extracellular ACh in freely moving rats treated with these drugs 20 min after seizure onset induced by a high dose of GD. Along with microdialysis, EEG activity was recorded and neuropathology assessed at 24 h. GD induced a marked increase of ACh, which peaked at 30 min post-exposure to 800% of control levels and then steadily decreased toward baseline levels. Approximately 40 min after treatment, only midazolam (10 mg/kg) and CPA (60 mg/kg) caused a significant reduction of ACh levels, with CPA reducing ACh levels more rapidly than midazolam. Both drugs facilitated a return to baseline levels at least 55 min after treatment. At 24 h, only animals treated with CPA (67%), midazolam (18%) and scopolamine (27%) exhibited seizure termination. While all treatments except for topiramate reduced neuropathology, CPA, midazolam and scopolamine showed the greatest reduction in pathology. Our results suggest that delayed treatment with CPA, midazolam, or scopolamine is effective at reducing GD-induced seizure activity and neuropathology, with CPA and midazolam capable of facilitating a reduction in GD-induced ACh elevation.
Kainate receptor-mediated synaptic transmissions in the adult rodent insular cortex.[Pubmed:22786952]
J Neurophysiol. 2012 Oct;108(7):1988-98.
Kainate (KA) receptors are expressed widely in the central nervous system and regulate both excitatory and inhibitory synaptic transmission. KA receptors play important roles in fear memory, anxiety, and pain. However, little is known about their function in synaptic transmission in the insular cortex (IC), a critical region for taste, memory, and pain. Using whole cell patch-clamp recordings, we have shown that KA receptors contribute to fast synaptic transmission in neurons in all layers of the IC. In the presence of the GABA(A) receptor antagonist picrotoxin, the NMDA receptor antagonist AP-5, and the selective AMPA receptor antagonist GYKI 53655, KA receptor-mediated excitatory postsynaptic currents (KA EPSCs) were revealed. We found that KA EPSCs are ~5-10% of AMPA/KA EPSCs in all layers of the adult mouse IC. Similar results were found in adult rat IC. KA EPSCs had a significantly slower rise time course and decay time constant compared with AMPA receptor-mediated EPSCs. High-frequency repetitive stimulations at 200 Hz significantly facilitated the summation of KA EPSCs. In addition, genetic deletion of GluK1 or GluK2 subunit partially reduced postsynaptic KA EPSCs, and exposure of GluK2 knockout mice to the selective GluK1 antagonist UBP 302 could significantly reduce the KA EPSCs. These data suggest that both GluK1 and GluK2 play functional roles in the IC. Our study may provide the synaptic basis for the physiology and pathology of KA receptors in the IC-related functions.
Kainate receptors mediate regulated exocytosis of secretory phospholipase A(2) in SH-SY5Y neuroblastoma cells.[Pubmed:22025073]
Neurosignals. 2012;20(2):72-85.
Secretory phospholipase A(2) (sPLA(2)) isoforms are widely expressed in the brain and spinal cord. Group IIA sPLA(2) (sPLA(2)-IIA) has been shown to stimulate exocytosis and release of neurotransmitters in neuroendocrine PC12 cells and neurons, suggesting a role of the enzyme in neuronal signaling and synaptic transmission. However, the mechanisms by which sPLA(2) is itself released, and a possible relation between glutamate receptors and sPLA(2) exocytosis, are unknown. This study was carried out to elucidate the effects of glutamate receptor agonists on exocytosis of sPLA(2)-IIA in transfected SH-SY5Y neuroblastoma cells. sPLA(2)-IIA enzyme was packaged in fusion-competent vesicles and released constitutively or upon stimulation, suggesting regulated secretion. The signal peptide of sPLA(2)-IIA is required for its vesicular localization and exocytosis. External application of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate (KA) induced vesicular exocytosis and release of sPLA(2)-IIA. UBP 302, a GluR5-specific KA receptor antagonist, abolished the effect of KA, confirming the role of KA receptors in mediating sPLA(2)-IIA secretion. Moreover, KA-induced sPLA(2)-IIA secretion is dependent on Ca(2+) and protein kinase C. Together, these findings provide evidence of a link between glutamate receptors and regulated sPLA(2) secretion in neurons that may play an important role in synaptic plasticity, pain transmission and neurodegenerative diseases.
In the developing hippocampus kainate receptors control the release of GABA from mossy fiber terminals via a metabotropic type of action.[Pubmed:21713663]
Adv Exp Med Biol. 2011;717:11-26.
Kainate receptors (KARs) are glutamate-gated ion channels assembled from various combinations of GluK1-GluK5 subunits with different physiological and pharmacological properties. In the hippocampus, KARs expressed at postsynaptic sites mediate a small component of excitatory postsynaptic currents while at presynaptic sites they exert a powerful control on transmitter release at both excitatory and inhibitory connections. KARs are developmentally regulated and play a key role in several developmental processes including neuronal migration, differentiation and synapse formation. Interestingly, they can signal through a canonical ionotropic pathway but also through a noncanonical modality involving pertussis toxin-sensitive G proteins and downstream signaling molecules.In this Chapter some of our recent data concerning the functional role of presynaptic KARs in regulation of transmitter release from immature mossy fiber terminals and in synaptic plasticity processes will be reviewed. Early in postnatal development, MFs release into their targeted neurons mainly GABA which is depolarizing and excitatory. Endogenous activation of GluK1 KARs localized on MF terminals by glutamate present in the extracellular space down regulates GABA release, leading sometimes to synapse silencing. The depressant effect of GluK1 on MF responses is mediated by a metabotropic process, sensitive to pertussis toxin and phospholipase C (PLC) along the transduction pathway downstream to G protein activation. Blocking PLC with the selective antagonist U73122, unmasks the potentiating effect of GluK1 on MF-evoked GABAergic currents, which probably depend on the ionotropic type of action of these receptors.In addition, GluK1 KARs dynamically regulate the direction of spike-time dependent plasticity, a particular form of Hebbian type of learning which consists in bidirectional modifications in synaptic strength according to the temporal order of pre and postsynaptic spiking. At immature MF-CA3 synapses pairing MF stimulation with postsynaptic spiking and vice versa induces long term depression of MF-evoked GABAergic currents. In the case of positive pairing synaptic depression can be switched into spike-time dependent potentiation by blocking GluK1 KARs with UBP 302. The depressant action exerted by GluK1 KARs on MF responses would prevent the excessive activation of the CA3 associative network by the excitatory action of GABA early in postnatal development.
Activation of iGluR5 kainate receptors inhibits neurogenic dural vasodilatation in an animal model of trigeminovascular activation.[Pubmed:19309356]
Br J Pharmacol. 2009 Jun;157(3):464-73.
BACKGROUND AND PURPOSE: Migraine is a disabling neurological disorder involving activation, or the perception of activation, of trigeminovascular afferents containing calcitonin gene-related peptide (CGRP). Released CGRP from peripheral trigeminal afferents causes dilatation of dural blood vessel, and this is used to measure trigeminal nerve activation. Kainate receptors with the GluR5 subunit (iGluR5, ionotropic glutamate receptor) are present in the trigeminal ganglion and may be involved in nociception. We investigated the possible involvement of prejunctional iGluR5 kainate receptors on CGRP release from trigeminal afferents. EXPERIMENTAL APPROACH: We used neurogenic dural vasodilatation, which involves reproducible vasodilatation in response to CGRP release after electrical stimulation of the dura mater surrounding the middle meningeal artery. The effects of the specific iGluR5 receptor antagonist UBP 302 and agonist (S)-(-)-5-iodowillardiine were investigated on neurogenic and CGRP-induced dural vasodilatation in rats, by using intravital microscopy. KEY RESULTS: Administration of 10 and 20 mg.kg(-1) of iodowillardiine inhibited electrically induced dural vessel dilatation, an effect blocked by pretreatment with 50 mg.kg(-1) UBP 302. Administration of the iGluR5 receptor antagonist UBP 302 alone had no significant effect. CGRP (1 mg.kg(-1))-induced dural vasodilatation was not inhibited by the iGluR5 receptor agonist iodowillardiine. CONCLUSIONS AND IMPLICATIONS: This study demonstrates that activation of the iGluR5 kainate receptors with the selective agonist iodowillardiine is able to inhibit neurogenic dural vasodilatation probably by inhibition of prejunctional release of CGRP from trigeminal afferents. Taken together with recent clinical studies the data reinforce CGRP mechanisms in primary headaches and demonstrate a novel role for kainate receptor modulation of trigeminovascular activation.
The limitations of diazepam as a treatment for nerve agent-induced seizures and neuropathology in rats: comparison with UBP302.[Pubmed:25157087]
J Pharmacol Exp Ther. 2014 Nov;351(2):359-72.
Exposure to nerve agents induces prolonged status epilepticus (SE), causing brain damage or death. Diazepam (DZP) is the current US Food and Drug Administration-approved drug for the cessation of nerve agent-induced SE. Here, we compared the efficacy of DZP with that of UBP302 [(S)-3-(2-carboxybenzyl)willardiine; an antagonist of the kainate receptors that contain the GluK1 subunit] against seizures, neuropathology, and behavioral deficits induced by soman in rats. DZP, administered 1 hour or 2 hours postexposure, terminated the SE, but seizures returned; thus, the total duration of SE within 24 hours after soman exposure was similar to (DZP at 1 hour) or longer than (DZP at 2 hours) that in the soman-exposed rats that did not receive the anticonvulsant. Compared with DZP, UBP302 stopped SE with a slower time course, but dramatically reduced the total duration of SE within 24 hours. Neuropathology and behavior were assessed in the groups that received anticonvulsant treatment 1 hour after exposure. UBP302, but not DZP, reduced neuronal degeneration in a number of brain regions, as well as neuronal loss in the basolateral amygdala and the CA1 hippocampal area, and prevented interneuronal loss in the basolateral amygdala. Anxiety-like behavior was assessed in the open field and by the acoustic startle response 30 days after soman exposure. The results showed that anxiety-like behavior was increased in the DZP-treated group and in the group that did not receive anticonvulsant treatment, but not in the UBP302-treated group. The results argue against the use of DZP for the treatment of nerve agent-induced seizures and brain damage and suggest that targeting GluK1-containing receptors is a more effective approach.
Synthesis and pharmacology of willardiine derivatives acting as antagonists of kainate receptors.[Pubmed:16302825]
J Med Chem. 2005 Dec 1;48(24):7867-81.
The natural product willardiine (8) is an AMPA receptor agonist while 5-iodowillardiine (10) is a selective kainate receptor agonist. In an attempt to produce antagonists of kainate and AMPA receptors analogues of willardiine with substituents at the N3 position of the uracil ring were synthesized. The N3-4-carboxybenzyl substituted analogue (38c) was found to be equipotent at AMPA and GLUK5-containing kainate receptors in the neonatal rat spinal cord. The N3-2-carboxybenzyl substituted analogue (38a) proved to be a potent and selective GLUK5 subunit containing kainate receptor antagonist when tested on native rat and human recombinant AMPA and kainate receptor subtypes. The GLUK5 kainate receptor antagonist activity was found to reside in the S enantiomer (44a) whereas the R enantiomer (44b) was almost inactive. 5-Iodo substitution of the uracil ring of 44a gave 45, which was found to have enhanced potency and selectivity for GLUK5.
Characterisation of UBP296: a novel, potent and selective kainate receptor antagonist.[Pubmed:15165833]
Neuropharmacology. 2004 Jul;47(1):46-64.
Willardiine derivatives with an N3-benzyl substituent bearing an acidic group have been synthesized with the aim of producing selective antagonists for GLUK5-containing kainate receptors. UBP296 was found to be a potent and selective antagonist of native GLUK5-containing kainate receptors in the spinal cord, with activity residing in the S enantiomer (UBP302). In cells expressing human kainate receptor subunits, UBP296 selectively depressed glutamate-induced calcium influx in cells containing GLUK5 in homomeric or heteromeric forms. In radioligand displacement binding studies, the willardiine analogues displaced [3H]kainate binding with IC50 values >100 microM at rat GLUK6, GLUK2 or GLUK6/GLUK2. An explanation of the GLUK5 selectivity of UBP296 was obtained using homology models of the antagonist bound forms of GLUK5 and GLUK6. In rat hippocampal slices, UBP296 reversibly blocked ATPA-induced depressions of synaptic transmission at concentrations subthreshold for affecting AMPA receptor-mediated synaptic transmission directly. UBP296 also completely blocked the induction of mossy fibre LTP, in medium containing 2 mM (but not 4 mM) Ca2+. These data provide further evidence for a role for GLUK5-containing kainate receptors in mossy fibre LTP. In conclusion, UBP296 is the most potent and selective antagonist of GLUK5-containing kainate receptors so far described.


