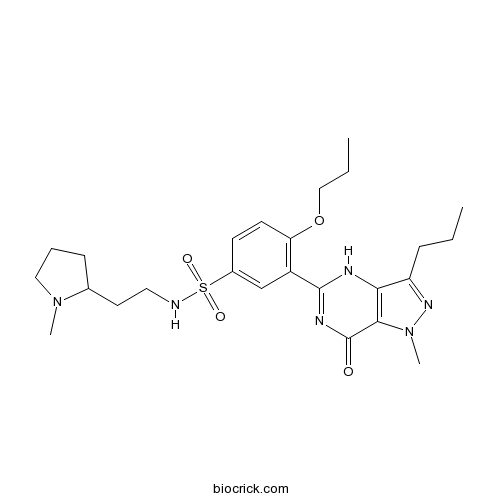
UdenafilCAS# 268203-93-6 |
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 268203-93-6 | SDF | Download SDF |
| PubChem ID | 6918523 | Appearance | Powder |
| Formula | C25H36N6O4S | M.Wt | 516.66 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
| Chemical Name | 3-(1-methyl-7-oxo-3-propyl-4H-pyrazolo[4,3-d]pyrimidin-5-yl)-N-[2-(1-methylpyrrolidin-2-yl)ethyl]-4-propoxybenzenesulfonamide | ||
| SMILES | CCCC1=NN(C2=C1NC(=NC2=O)C3=C(C=CC(=C3)S(=O)(=O)NCCC4CCCN4C)OCCC)C | ||
| Standard InChIKey | IYFNEFQTYQPVOC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H36N6O4S/c1-5-8-20-22-23(31(4)29-20)25(32)28-24(27-22)19-16-18(10-11-21(19)35-15-6-2)36(33,34)26-13-12-17-9-7-14-30(17)3/h10-11,16-17,26H,5-9,12-15H2,1-4H3,(H,27,28,32) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Udenafil Dilution Calculator

Udenafil Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9355 mL | 9.6775 mL | 19.3551 mL | 38.7102 mL | 48.3877 mL |
| 5 mM | 0.3871 mL | 1.9355 mL | 3.871 mL | 7.742 mL | 9.6775 mL |
| 10 mM | 0.1936 mL | 0.9678 mL | 1.9355 mL | 3.871 mL | 4.8388 mL |
| 50 mM | 0.0387 mL | 0.1936 mL | 0.3871 mL | 0.7742 mL | 0.9678 mL |
| 100 mM | 0.0194 mL | 0.0968 mL | 0.1936 mL | 0.3871 mL | 0.4839 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Debilon
Catalog No.:BCN7696
CAS No.:26808-51-5
- Indapamide
Catalog No.:BCC4788
CAS No.:26807-65-8
- Dehydrodiisoeugenol
Catalog No.:BCN1240
CAS No.:2680-81-1
- Xanthatin
Catalog No.:BCN5150
CAS No.:26791-73-1
- Robtein
Catalog No.:BCN4658
CAS No.:2679-65-4
- Amoxicillin
Catalog No.:BCC4625
CAS No.:26787-78-0
- Alibendol
Catalog No.:BCC4758
CAS No.:26750-81-2
- Perivine
Catalog No.:BCN2583
CAS No.:2673-40-7
- Canertinib
Catalog No.:BCN2172
CAS No.:267243-28-7
- [Nphe1]Nociceptin(1-13)NH2
Catalog No.:BCC5739
CAS No.:267234-08-2
- Picraline
Catalog No.:BCN4762
CAS No.:2671-32-1
- Boc-Glycinol
Catalog No.:BCC3093
CAS No.:26690-80-2
- Coronalolide methyl ester
Catalog No.:BCN5151
CAS No.:268214-50-2
- Coronalolide
Catalog No.:BCN5152
CAS No.:268214-51-3
- Coronalolic acid
Catalog No.:BCN5153
CAS No.:268214-52-4
- Harringtonine
Catalog No.:BCN6794
CAS No.:26833-85-2
- Homoharringtonine
Catalog No.:BCN4958
CAS No.:26833-87-4
- Triptohypol F
Catalog No.:BCN5154
CAS No.:268541-26-0
- Penfluridol
Catalog No.:BCC4696
CAS No.:26864-56-2
- 1,3-Bis[2-(4-aminophenyl)-2-propyl]benzene
Catalog No.:BCC8419
CAS No.:2687-27-6
- Isomahanimbine
Catalog No.:BCN3175
CAS No.:26871-46-5
- 6-Hydroxybenzofuran-2(3H)-one
Catalog No.:BCN5155
CAS No.:2688-49-5
- Oxysophocarpine
Catalog No.:BCN5156
CAS No.:26904-64-3
- 2(-4-Chloro-3-hydroxy-1-butynyl)-5-1,(3-pentadiynyl)thiophene
Catalog No.:BCN1465
CAS No.:26905-70-4
Population pharmacokinetic analysis to recommend the optimal dose of udenafil in patients with mild and moderate hepatic impairment.[Pubmed:27084997]
Br J Clin Pharmacol. 2016 Aug;82(2):389-98.
AIMS: The aim of this study was to develop a population pharmacokinetic (PK) model of Udenafil and its active metabolite, DA-8164, in healthy subjects and patients with hepatic impairment (HI) and to estimate the optimal dosing recommendations for patients with HI. METHODS: An open label, three parallel group, age and weight matched control study was conducted in 18 volunteers, six healthy subjects (n = 6) and patients with mild (Child-Pugh class A, n = 6) and moderate HI (Child-Pugh class B, n = 6). Serial blood samples were collected for up to 72 h after a single administration of Udenafil 100 mg. A population PK model was developed using non-linear mixed effects modelling (nonmem, ver. 7.2). The simulated data from the final PK model and original data of healthy subjects were compared to identify the optimal dose for patients with HI. RESULTS: A two compartment model for both Udenafil and DA-8164 best described the data. Prothrombin time on metabolic clearance of Udenafil to DA-8164 was included in the final model as a covariate. Compared with the AUC(0,tlast ) value after administration of Udenafil 100 mg to healthy subjects, the geometric mean ratios (95% confidence interval) after 100 mg and 75 mg Udenafil administration were 1.21 (1.10, 1.32) and 0.74 (0.67, 0.81) in patients with mild HI, respectively. Meanwhile, those were 1.55 (1.43, 1.67) and 1.02 (0.92, 1.12) in patients with moderate HI, respectively. CONCLUSIONS: This study suggests that the recommended doses of Udenafil are 100 mg and 75 mg in patients with mild and moderate HI, respectively.
A Phase 3 Study to Evaluate the 1-Year Efficacy and Safety of Udenafil 75 mg Once Daily in Patients With Erectile Dysfunction.[Pubmed:27319276]
J Sex Med. 2016 Aug;13(8):1263-9.
INTRODUCTION: Once-daily administration of phosphodiesterase type 5 inhibitors has been shown to correct erectile dysfunction (ED). AIM: To evaluate the long-term efficacy and safety after once-daily oral administration of Udenafil 75 mg in men with ED. METHODS: This clinical trial was an open-label, fixed-dose, 24-week extension study (DA8159_EDDL_III) of a 24-week double-blinded efficacy and safety study of once-daily Udenafil (parent study: DA8159_EDD_III). Subjects received Udenafil 75 mg once daily for 24 weeks during this extension study, and the follow-up visit occurred during the 4-week ED treatment-free period. MAIN OUTCOME MEASURES: Subjects were asked to complete the International Index of Erectile Function questionnaire and the Global Assessment Questionnaire at the 24-week extension and after the 4-week ED treatment-free period, and the development of adverse drug reactions was investigated. RESULTS: In total, 302 subjects were enrolled in this extension study. Improvement was shown with an increased erectile function (EF) domain score compared with baseline (14.60 +/- 4.57) at extension week 48 (23.98 +/- 5.44) and a slight increase in EF domain score compared with the last time point (week 24) of the parent study (P < .001). The Global Assessment Questionnaire showed a high improvement rate of 95.4% at the extension 48-week time point. For shift to normal, almost half the subjects (45.1%) recovered "normal" EF, and 14.2% of subjects reported normal erections after the 4-week ED treatment-free period. The occurrence rate of adverse drug reactions was 8%, which consisted mainly of flushing and headache. CONCLUSION: Once-daily dosing of Udenafil 75 mg showed excellent efficacy and safety with long-term administration and allowed a more spontaneous sexual life.
The Effect of Age on the Pharmacokinetics of Udenafil in Healthy Subjects.[Pubmed:27006150]
J Clin Pharmacol. 2016 Nov;56(11):1372-1377.
Udenafil, a cyclic guanosine monophosphate-specific phosphodiesterase type 5 inhibitor, has been developed to treat erectile dysfunction. We evaluated the effect of age on the pharmacokinetics and tolerability of Udenafil. A single-center, open-label, parallel-group phase 1 study was conducted in healthy adult subjects who took a single oral dose of Udenafil (100 mg). The pharmacokinetics and tolerability of Udenafil were compared between 12 healthy young men (21-27 years) and 12 healthy elderly men (65-78 years). Serial blood and urine samples were collected for up to 60 and 48 hours after dosing. The plasma concentrations of Udenafil and its major metabolite, DA-8164, were analyzed using a validated liquid chromatography-tandem mass spectrometry method. The mean Cmax of Udenafil tended to be slightly less (214.0 vs 292.8 mug/L) in the elderly compared with the young (GMR, 68.9; 95% CI, 48.9-97.1); however, the AUC did not differ between the groups (1858.8 vs 2100.6 mug.h/L; GMR, 84.6; 95% CI, 66.1-108.4). The mean t1/2 was prolonged by approximately 5 hours in the elderly (P < .05). The clearance and metabolic AUC ratio did not differ between the elderly and young. In terms of tolerability, all adverse events were mild, and all subjects recovered without additional therapy. The systemic exposure of elderly subjects to Udenafil appears to be comparable to or slightly less than that of young healthy subjects. Based on our pharmacokinetic comparisons, Udenafil dose adjustment is unlikely to be required in the elderly population.


