VeratraldehydeCAS# 120-14-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 120-14-9 | SDF | Download SDF |
| PubChem ID | 8419 | Appearance | Powder |
| Formula | C9H10O3 | M.Wt | 166.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
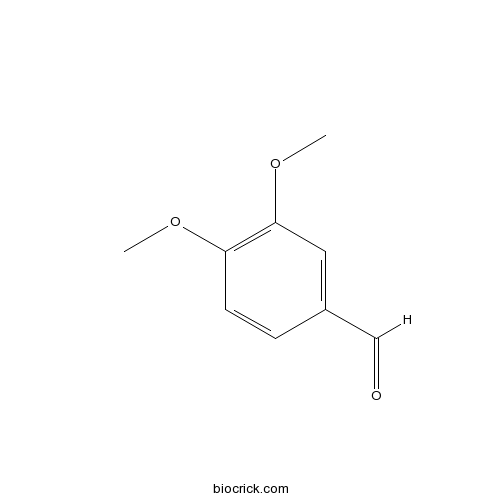
| Chemical Name | 3,4-dimethoxybenzaldehyde | ||
| SMILES | COC1=C(C=C(C=C1)C=O)OC | ||
| Standard InChIKey | WJUFSDZVCOTFON-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H10O3/c1-11-8-4-3-7(6-10)5-9(8)12-2/h3-6H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Veratraldehyde as a corrosion inhibitor for Zinc in different acid medium. Veratraldehyde was reduced by aryl-alcohol dehydrogenase to their corresponding alcohols, which was oxidized by aryl-alcohol oxidase, producing H2O2. |
| In vitro | Use of benzo analogs to enhance antimycotic activity of kresoxim methyl for control of aflatoxigenic fungal pathogens.[Pubmed: 24639673]Front Microbiol. 2014 Mar 7;5:87.The aim of this study was to examine two benzo analogs, octylgallate (OG) and Veratraldehyde (VT), as antifungal agents against strains of Aspergillus parasiticus and A.flavus (toxigenic or atoxigenic). Veratraldehyde as Corrosion Inhibitor for Zinc in Different Acid Mediu[Reference: WebLink]Der Pharma Chemica, 2010,2(6):295.
|
| Structure Identification | Phys Chem Chem Phys. 2010 Jul 21;12(27):7603-11.Photoenhanced degradation of veratraldehyde upon the heterogeneous ozone reactions.[Pubmed: 20502834]Light-induced heterogeneous reactions between gas-phase ozone and Veratraldehyde adsorbed on silica particles were performed. Appl Environ Microbiol. 1994 Aug;60(8):2811-7.Anisaldehyde and Veratraldehyde Acting as Redox Cycling Agents for H(2)O(2) Production by Pleurotus eryngii.[Pubmed: 16349349]
|

Veratraldehyde Dilution Calculator

Veratraldehyde Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.0168 mL | 30.0842 mL | 60.1685 mL | 120.3369 mL | 150.4212 mL |
| 5 mM | 1.2034 mL | 6.0168 mL | 12.0337 mL | 24.0674 mL | 30.0842 mL |
| 10 mM | 0.6017 mL | 3.0084 mL | 6.0168 mL | 12.0337 mL | 15.0421 mL |
| 50 mM | 0.1203 mL | 0.6017 mL | 1.2034 mL | 2.4067 mL | 3.0084 mL |
| 100 mM | 0.0602 mL | 0.3008 mL | 0.6017 mL | 1.2034 mL | 1.5042 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Scoparone
Catalog No.:BCN6088
CAS No.:120-08-1
- Sulfuretin
Catalog No.:BCN4725
CAS No.:120-05-8
- Unedone
Catalog No.:BCN6759
CAS No.:1199815-09-2
- INT-777
Catalog No.:BCC5390
CAS No.:1199796-29-6
- 1,2-Didehydrotanshinone IIA
Catalog No.:BCN3143
CAS No.:119963-50-7
- Peroxy Orange 1
Catalog No.:BCC6336
CAS No.:1199576-10-7
- 7-O-Methylmorroniside
Catalog No.:BCN7293
CAS No.:119943-46-3
- CGRP 8-37 (human)
Catalog No.:BCC5724
CAS No.:119911-68-1
- Posaconazole hydrate
Catalog No.:BCC4234
CAS No.:1198769-38-8
- Esomeprazole magnesium salt
Catalog No.:BCC5430
CAS No.:1198768-91-0
- Phyperunolide E
Catalog No.:BCN7292
CAS No.:1198400-52-0
- Coptisine sulfate
Catalog No.:BCN2286
CAS No.:1198398-71-8
- Tropine benzilate
Catalog No.:BCN1921
CAS No.:3736-36-5
- Clorofene
Catalog No.:BCC8919
CAS No.:120-32-1
- 3-Amino-4-methoxybenzanilide
Catalog No.:BCC8613
CAS No.:120-35-4
- Desoxyanisoin
Catalog No.:BCN2264
CAS No.:120-44-5
- Ethylparaben
Catalog No.:BCN6094
CAS No.:120-47-8
- Benzyl benzoate
Catalog No.:BCN8521
CAS No.:120-51-4
- Isosafrole
Catalog No.:BCC3976
CAS No.:120-58-1
- 2'-Methylacetanilide
Catalog No.:BCC8581
CAS No.:120-66-1
- N,N'-Bis(salicylidene)-1,3-propanediamine
Catalog No.:BCC9063
CAS No.:120-70-7
- 1,2-Benzenediol
Catalog No.:BCN6103
CAS No.:120-80-9
- Dichlorphenamide
Catalog No.:BCC3761
CAS No.:120-97-8
- Donepezil HCl
Catalog No.:BCC4569
CAS No.:120011-70-3
Combinatorial evaluation of laccase-mediator system in the oxidation of veratryl alcohol.[Pubmed:23132490]
Biotechnol Lett. 2013 Feb;35(2):225-31.
Laccases play an important role in the biological break down of lignin and have great potential in the deconstruction of lignocellulosic feedstocks. We examined 16 laccases, both commercially prepared and crude extracts, for their ability to oxidize veratryl alcohol in the presence of various solvents and mediators. Screening revealed complete conversion of veratryl alcohol to Veratraldehyde catalyzed by a crude preparation of the laccase from Trametes versicolor ATCC 11235 and the mediator TEMPO in 20 % (v/v) tert-butanol.
Use of benzo analogs to enhance antimycotic activity of kresoxim methyl for control of aflatoxigenic fungal pathogens.[Pubmed:24639673]
Front Microbiol. 2014 Mar 7;5:87.
The aim of this study was to examine two benzo analogs, octylgallate (OG) and Veratraldehyde (VT), as antifungal agents against strains of Aspergillus parasiticus and A.flavus (toxigenic or atoxigenic). Both toxigenic and atoxigenic strains used were capable of producing kojic acid, another cellular secondary product. A. fumigatus was used as a genetic model for this study. When applied independently, OG exhibits considerably higher antifungal activity compared to VT. The minimum inhibitory concentrations (MICs) of OG were 0.3-0.5 mM, while that of VT were 3.0-5.0 mM in agar plate-bioassays. OG or VT in concert with the fungicide kresoxim methyl (Kre-Me; strobilurin) greatly enhanced sensitivity of Aspergillus strains to Kre-Me. The combination with OG also overcame the tolerance of A. fumigatus mitogen-activated protein kinase (MAPK) mutants to Kre-Me. The degree of compound interaction resulting from chemosensitization of the fungi by OG was determined using checkerboard bioassays, where synergistic activity greatly lowered MICs or minimum fungicidal concentrations. However, the control chemosensitizer benzohydroxamic acid, an alternative oxidase inhibitor conventionally applied in concert with strobilurin, did not achieve synergism. The level of antifungal or chemosensitizing activity was also "compound-strain" specific, indicating differential susceptibility of tested strains to OG or VT, and/or heat stress. Besides targeting the antioxidant system, OG also negatively affected the cell wall-integrity pathway, as determined by the inhibition of Saccharomyces cerevisiae cell wall-integrity MAPK pathway mutants. We concluded that certain benzo analogs effectively inhibit fungal growth. They possess chemosensitizing capability to increase efficacy of Kre-Me and thus, could reduce effective dosages of strobilurins and alleviate negative side effects associated with current antifungal practices. OG also exhibits moderate antiaflatoxigenic activity.
Anisaldehyde and Veratraldehyde Acting as Redox Cycling Agents for H(2)O(2) Production by Pleurotus eryngii.[Pubmed:16349349]
Appl Environ Microbiol. 1994 Aug;60(8):2811-7.
The existence of a redox cycle leading to the production of hydrogen peroxide (H(2)O(2)) in the white rot fungus Pleurotus eryngii has been confirmed by incubations of 10-day-old mycelium with veratryl (3,4-dimethoxybenzyl) and anisyl (4-methoxybenzyl) compounds (alcohols, aldehydes, and acids). Veratraldehyde and anisaldehyde were reduced by aryl-alcohol dehydrogenase to their corresponding alcohols, which were oxidized by aryl-alcohol oxidase, producing H(2)O(2). Veratric and anisic acids were incorporated into the cycle after their reduction, which was catalyzed by aryl-aldehyde dehydrogenase. With the use of different initial concentrations of either veratryl alcohol, Veratraldehyde, or veratric acid (0.5 to 4.0 mM), around 94% of Veratraldehyde and 3% of veratryl alcohol (compared with initial concentrations) and trace amounts of veratric acid were found when equilibrium between reductive and oxidative activities had been reached, regardless of the initial compound used. At concentrations higher than 1 mM, veratric acid was not transformed, and at 1.0 mM, it produced a negative effect on the activities of aryl-alcohol oxidase and both dehydrogenases. H(2)O(2) levels were proportional to the initial concentrations of veratryl compounds (around 0.5%), and an equilibrium between aryl-alcohol oxidase and an unknown H(2)O(2)-reducing system kept these levels steady. On the other hand, the concomitant production of the three above-mentioned enzymes during the active growth phase of the fungus was demonstrated. Finally, the possibility that anisaldehyde is the metabolite produced by P. eryngii for the maintenance of this redox cycle is discussed.
Photoenhanced degradation of veratraldehyde upon the heterogeneous ozone reactions.[Pubmed:20502834]
Phys Chem Chem Phys. 2010 Jul 21;12(27):7603-11.
Light-induced heterogeneous reactions between gas-phase ozone and Veratraldehyde adsorbed on silica particles were performed. At an ozone mixing ratio of 250 ppb, the loss of Veratraldehyde largely increased from 1.81 x 10(-6) s(-1) in the dark to 2.54 x 10(-5) s(-1) upon exposure to simulated sunlight (lambda > 300 nm). The observed rates of degradation exhibited linear dependence with the ozone in the dark ozonolysis experiments which change in the non-linear Langmuir-Hinshelwood dependence in the experiments with simultaneous ozone and light exposure of the coated particles. When the coated silica particles were exposed only to simulated sunlight in absence of ozone the loss of Veratraldehyde was about three times higher i.e. 5.97 x 10(-6) s(-1) in comparison to the ozonolysis experiment under dark conditions at 250 ppb ozone mixing ratio, 1.81 x 10(-6) s(-1).These results clearly show that the most important loss of Veratraldehyde occurs under simultaneous ozone and light exposure of the coated silica particles. The main identified product in the heterogeneous reactions between gaseous ozone and adsorbed Veratraldehyde under dark conditions and in presence of light was veratric acid.Carbon yields of veratric acid were calculated and the obtained results indicated that at low ozone mixing ratio (250 ppb) the carbon yield obtained under dark conditions is 70% whereas the carbon yield obtained in the experiments with simultaneous ozone and light exposure of the coated particles is 40%. In both cases the carbon yield of veratric acid exponentially decayed leading to the plateau ( approximately 35% of carbon yield) at an ozone mixing ratio of 6 ppm. Two reaction products i.e. 3-hydroxy-4-methoxybenzoic acid and 4-hydroxy-3-methoxybenzoic acid were identified (confirmed with the standards) only in the experiments performed under simultaneous ozonolysis and light irradiation of the particles.


