ZederoneCAS# 7727-79-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 7727-79-9 | SDF | Download SDF |
| PubChem ID | 101286196 | Appearance | Powder |
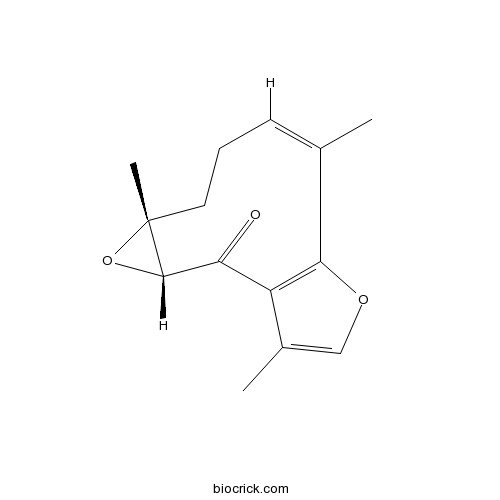
| Formula | C15H18O3 | M.Wt | 246.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3R,5R,8Z)-5,9,14-trimethyl-4,12-dioxatricyclo[9.3.0.03,5]tetradeca-1(11),8,13-trien-2-one | ||
| SMILES | CC1=CCCC2(C(O2)C(=O)C3=C(C1)OC=C3C)C | ||
| Standard InChIKey | CVIVANCKIBYAOP-HBXAWUERSA-N | ||
| Standard InChI | InChI=1S/C15H18O3/c1-9-5-4-6-15(3)14(18-15)13(16)12-10(2)8-17-11(12)7-9/h5,8,14H,4,6-7H2,1-3H3/b9-5-/t14-,15+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Zederone has anti-bacterial activity,it inhibits gram-positive bacteria activity. Zederone induces hepatotoxicity implicated the induction of Cyps, which leads to the formation of biological reactive metabolites and that cause the oxidative stress and liver cell injuries. |
| Targets | TNF-α | Nrf2 | NADPH-oxidase | P450 (e.g. CYP17) | Antifection |
| In vivo | Zederone, a sesquiterpene from Curcuma elata Roxb, is hepatotoxic in mice.[Pubmed: 24082031]Int J Toxicol. 2013 Nov-Dec;32(6):454-62.The present study aimed to investigate the hepatotoxicity of Zederone isolated from Curcuma elata in mice. |
| Kinase Assay | Interactions of sesquiterpenes zederone and germacrone with the human cytochrome P450 system.[Pubmed: 23850985]Toxicol In Vitro. 2013 Sep;27(6):2005-12.Misclassification of Curcuma species (family Zingiberaceae) may lead to unwanted human exposure to Curcuma elata sesquiterpenes Zederone and germacrone which have caused hepatotoxicity and changes in CYP expression in laboratory animals. |

Zederone Dilution Calculator

Zederone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0601 mL | 20.3004 mL | 40.6009 mL | 81.2018 mL | 101.5022 mL |
| 5 mM | 0.812 mL | 4.0601 mL | 8.1202 mL | 16.2404 mL | 20.3004 mL |
| 10 mM | 0.406 mL | 2.03 mL | 4.0601 mL | 8.1202 mL | 10.1502 mL |
| 50 mM | 0.0812 mL | 0.406 mL | 0.812 mL | 1.624 mL | 2.03 mL |
| 100 mM | 0.0406 mL | 0.203 mL | 0.406 mL | 0.812 mL | 1.015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Abyssinone V
Catalog No.:BCN6825
CAS No.:77263-11-7
- Erythrabyssin II
Catalog No.:BCN4828
CAS No.:77263-06-0
- MG 624
Catalog No.:BCC7028
CAS No.:77257-42-2
- Carboxyatractyloside
Catalog No.:BCN2880
CAS No.:77228-71-8
- Nefiracetam
Catalog No.:BCC4504
CAS No.:77191-36-7
- Rheediaxanthone A
Catalog No.:BCN7411
CAS No.:77181-97-6
- Acetylaconitine
Catalog No.:BCN2407
CAS No.:77181-26-1
- Delta-Caesalpin
Catalog No.:BCN6698
CAS No.:7716-14-5
- Spiraline
Catalog No.:BCN2112
CAS No.:77156-25-3
- Spiracine
Catalog No.:BCN2095
CAS No.:77156-24-2
- Spiranine
Catalog No.:BCN2094
CAS No.:77156-23-1
- 6alpha-Hydroxycleroda-3,13-dien-16,15-olid-18-oic acid
Catalog No.:BCN1359
CAS No.:771493-42-6
- Fmoc-Nle-OH.
Catalog No.:BCC3298
CAS No.:77284-32-3
- Edelfosine
Catalog No.:BCC7537
CAS No.:77286-66-9
- Hirsutine
Catalog No.:BCN2758
CAS No.:7729-23-9
- SL 0101-1
Catalog No.:BCC8086
CAS No.:77307-50-7
- Hyuganin D
Catalog No.:BCN7679
CAS No.:77331-76-1
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
- Triacetylpseurotin A
Catalog No.:BCN6916
CAS No.:77353-57-2
- Drimiopsin C
Catalog No.:BCN4325
CAS No.:773850-90-1
- Drimiopsin D
Catalog No.:BCN4326
CAS No.:773850-91-2
- Sesartemin
Catalog No.:BCN4779
CAS No.:77394-27-5
- Episesartemin A
Catalog No.:BCN7239
CAS No.:77449-31-1
- 5-Androsten-3β-ol-17-one ethyleneketal
Catalog No.:BCC8738
CAS No.:7745-40-6
Interactions of sesquiterpenes zederone and germacrone with the human cytochrome P450 system.[Pubmed:23850985]
Toxicol In Vitro. 2013 Sep;27(6):2005-12.
Misclassification of Curcuma species (family Zingiberaceae) may lead to unwanted human exposure to Curcuma elata sesquiterpenes Zederone and germacrone which have caused hepatotoxicity and changes in CYP expression in laboratory animals. We investigated how these compounds interact with the human cytochrome P450 (CYP) system, in order to evaluate their potential for human liver toxicity and herb-drug interactions. We found that both sesquiterpenes (1-30 muM) greatly induced expression of CYP2B6 and CYP3A4 but not CYP1A2 mRNAs in human primary hepatocytes (HPHs). This induction profile correlated with activation of constitutive androstane and pregnane X receptors. Cytotoxicity was also observed in exposed HPHs. CYP inhibition studies with pooled human liver microsomes (HLMs) indicated that Zederone and germacrone moderately inhibited CYP2B6 and CYP3A4 activities in vitro, with IC50 values below 10 muM. When Zederone was incubated with HLMs and NADPH, one di-epoxide metabolite was formed and by using glutathione trapping, five epoxide-derived conjugates were detected. Germacrone produced two oxidized metabolites and four glutathione conjugates. The results suggest that enzymes in HLMs convert sesquiterpenes into reactive, electrophilic compounds which may be causative for the reported liver injuries. These findings provide insight on the safety and drug-herb interactions of the Curcuma species.
Zederone, a sesquiterpene from Curcuma elata Roxb, is hepatotoxic in mice.[Pubmed:24082031]
Int J Toxicol. 2013 Nov-Dec;32(6):454-62.
The present study aimed to investigate the hepatotoxicity of Zederone isolated from Curcuma elata in mice. Adult male mice were intraperitoneally injected with a single dose of Zederone (50-300 mg/kg body weight [BW]). Twenty-four hours after the injection, Zederone induced liver enlargement with scattered white foci over the organ. The medium lethal dose (LD(5)(0)) value at 24 hours of Zederone was approximately 223 mg/kg BW. Hepatic centrilobular necrosis with marked increases in plasma alanine transaminase activity and total bilirubin levels was observed. Zederone at a dose of 200 mg/kg BW markedly decreased the activity of superoxide dismutase and the hepatic glutathione content, whereas the activity of catalase was not altered. The compound at this dose also increased the messenger RNA (mRNA) expression of Cyp2b10 and Cyp3a11, which are the main drug-metabolizing enzymes in the liver. The mRNA expression of proinflammatory cytokine tumor necrosis factor alpha was increased. The nuclear factor-E2-related factor 2 protein, which is the transcription factor regulating the antioxidant gene expression, was decreased. The histopathology of massive hepatic centrilobular necrosis with an increase in the expression of cytochrome P450 (Cyp) suggests that the possible potentiation of Zederone-induced hepatotoxicity implicated the induction of Cyps, which leads to the formation of biological reactive metabolites and that cause the oxidative stress and liver cell injuries.


