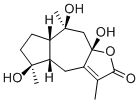
Zedoarolide BCAS# 213833-36-4 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 213833-36-4 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C15H22O5 | M.Wt | 282.37 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Zedoarolide B Dilution Calculator

Zedoarolide B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5415 mL | 17.7073 mL | 35.4145 mL | 70.8291 mL | 88.5363 mL |
| 5 mM | 0.7083 mL | 3.5415 mL | 7.0829 mL | 14.1658 mL | 17.7073 mL |
| 10 mM | 0.3541 mL | 1.7707 mL | 3.5415 mL | 7.0829 mL | 8.8536 mL |
| 50 mM | 0.0708 mL | 0.3541 mL | 0.7083 mL | 1.4166 mL | 1.7707 mL |
| 100 mM | 0.0354 mL | 0.1771 mL | 0.3541 mL | 0.7083 mL | 0.8854 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Trijugin B
Catalog No.:BCX0286
CAS No.:1233534-43-4
- Triumbelletin 7-O-glucoside
Catalog No.:BCX0285
CAS No.:2222584-03-2
- Ubine
Catalog No.:BCX0284
CAS No.:34469-09-5
- 3,4-Dihydroxy-N-(2-hydroxy-2-phenylethyl)benzamide
Catalog No.:BCX0283
CAS No.:1916734-50-3
- p-Hydroxyphenylethyl p-coumarate
Catalog No.:BCX0282
CAS No.:149030-02-4
- Trijugin A
Catalog No.:BCX0281
CAS No.:1233534-41-2
- Taxifolin 6-C-glucoside
Catalog No.:BCX0280
CAS No.:112494-39-0
- Pteroside T
Catalog No.:BCX0279
CAS No.:62043-51-0
- Aromadendrin 6-C-glucoside
Catalog No.:BCX0278
CAS No.:112494-34-5
- Trijugin C
Catalog No.:BCX0277
CAS No.:1233534-45-6
- Lup-20(29)-ene-2α,3α-diol
Catalog No.:BCX0276
CAS No.:55476-83-0
- 6-(4-Hydroxyphenyl)-2-methylhept-2-en-4-one
Catalog No.:BCX0275
CAS No.:949081-06-5
- Zedoalactone B
Catalog No.:BCX0288
CAS No.:170384-81-3
- 5-Deoxytrijugin C
Catalog No.:BCX0289
CAS No.:135971-83-4
- Zedoalactone A
Catalog No.:BCX0290
CAS No.:170384-82-4
- 5-Hydroxynoracronycine
Catalog No.:BCX0291
CAS No.:27067-70-5
- 5α,6α-Epoxy-3β-hydroxymegastigm-7-en-9-one
Catalog No.:BCX0292
CAS No.:50281-42-0
- Trijugin H
Catalog No.:BCX0293
CAS No.:1233534-55-8
- 24-O-Acetyllycoclavanol
Catalog No.:BCX0294
CAS No.:13956-53-1
- Racemoflavone
Catalog No.:BCX0295
CAS No.:106055-12-3
- Trijugin G
Catalog No.:BCX0296
CAS No.:1233534-53-6
- Isoorientin 3'-O-glucoside
Catalog No.:BCX0297
CAS No.:53098-95-6
- 2-Ethyl-3-methylmaleimide
Catalog No.:BCX0298
CAS No.:20189-42-8
- Balsaminone B
Catalog No.:BCX0299
CAS No.:213271-56-8
Bioactive chemical constituents from Curcuma caesia Roxb. rhizomes and inhibitory effect of curcuzederone on the migration of triple-negative breast cancer cell line MDA-MB-231.[Pubmed:31726856]
Nat Prod Res. 2021 Sep;35(18):3166-3170.
Rhizomes of Curcuma caesia are traditionally used to treat cancer in India. The aim is to isolate chemical constituents from C. caesia rhizomes through bioassay-guided fractionation. The extract, hexanes and chloroform fractions showed effect on MCF-7 and MDA-MB-231cells in cell viability assay. The chromatographic separation afforded germacrone (1), zerumbone (2), furanodienone (3), curzerenone (4), curcumenol (5), zederone (6), curcumenone (7), dehydrocurdione (8) from hexanes fraction and curcuminol G (9), curcuzederone (10), (1S, 10S), (4S,5S)-germacrone-1 (10), 4-diepoxide (11), wenyujinin B (12), alismoxide (13), aerugidiol (14), Zedoarolide B (15), zedoalactone B (16), zedoarondiol (17), isozedoarondiol (18) from chloroform fraction. This is first report of compounds 2, 9-13, 15-18 from C. caesia. The study demonstrated compounds 1-4 and 10 are the bioactive compounds. The effect of curcuzederone (10) on MDA-MB-231 cell migration showed significant inhibition in scratch and Transwell migration assays. The results revealed that curcuzederone could be a promising drug to treat cancer.
Three new guaiane sesquiterpene lactones from rhizomes of Curcuma wenyujin.[Pubmed:23679107]
J Asian Nat Prod Res. 2013 Jul;15(7):723-30.
Three new guaiane sesquiterpene lactones (4S)-4-hydroxy-gweicurculactone (1), zedoalactone G (2), and (1R, 4R, 5S, 10S)-zedoalactone B (3), and three known guaiane sesquiterpene lactones, including Zedoarolide B (4), zedoalactone B (5), and a new natural product (+)-zedoalactone A (6), were isolated from the rhizomes of Curcuma wenyujin Y.H. Chen et C. Ling. The structures were elucidated by spectroscopic methods including 1D and 2D NMR and HR-ESI-MS. The absolute configuration of 2 was determined via the calculated electronic circular dichroism (ECD), whereas the absolute configurations of 1 and 3 were determined via the ECD data of the [Rh2(OCOCF3)4] complex and [Mo2(OAc)4] complex, respectively. The inhibitory effects of compounds 1-6 on nitric oxide production in lipopolysaccharide-activated macrophages were evaluated. All of them exhibited weak anti-inflammatory activity.


