5-HydroxynoracronycineCAS# 27067-70-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 27067-70-5 | SDF | Download SDF |
| PubChem ID | 5378702 | Appearance | Orange powder |
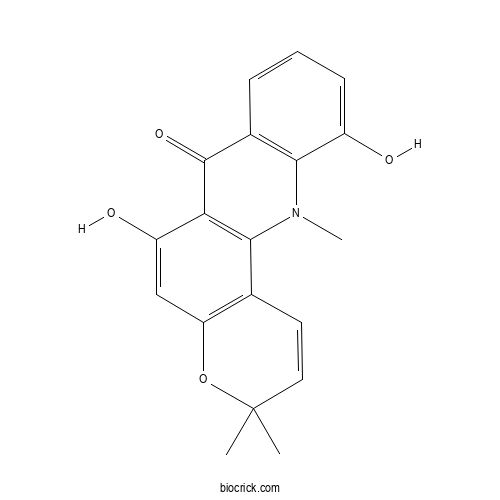
| Formula | C19H17NO4 | M.Wt | 323.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 6,11-dihydroxy-3,3,12-trimethylpyrano[2,3-c]acridin-7-one | ||
| SMILES | CC1(C=CC2=C(O1)C=C(C3=C2N(C4=C(C3=O)C=CC=C4O)C)O)C | ||
| Standard InChIKey | JZQDCDLYNFZBIG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H17NO4/c1-19(2)8-7-10-14(24-19)9-13(22)15-17(10)20(3)16-11(18(15)23)5-4-6-12(16)21/h4-9,21-22H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

5-Hydroxynoracronycine Dilution Calculator

5-Hydroxynoracronycine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0931 mL | 15.4655 mL | 30.931 mL | 61.862 mL | 77.3276 mL |
| 5 mM | 0.6186 mL | 3.0931 mL | 6.1862 mL | 12.3724 mL | 15.4655 mL |
| 10 mM | 0.3093 mL | 1.5466 mL | 3.0931 mL | 6.1862 mL | 7.7328 mL |
| 50 mM | 0.0619 mL | 0.3093 mL | 0.6186 mL | 1.2372 mL | 1.5466 mL |
| 100 mM | 0.0309 mL | 0.1547 mL | 0.3093 mL | 0.6186 mL | 0.7733 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Zedoalactone A
Catalog No.:BCX0290
CAS No.:170384-82-4
- 5-Deoxytrijugin C
Catalog No.:BCX0289
CAS No.:135971-83-4
- Zedoalactone B
Catalog No.:BCX0288
CAS No.:170384-81-3
- Zedoarolide B
Catalog No.:BCX0287
CAS No.:213833-36-4
- Trijugin B
Catalog No.:BCX0286
CAS No.:1233534-43-4
- Triumbelletin 7-O-glucoside
Catalog No.:BCX0285
CAS No.:2222584-03-2
- Ubine
Catalog No.:BCX0284
CAS No.:34469-09-5
- 3,4-Dihydroxy-N-(2-hydroxy-2-phenylethyl)benzamide
Catalog No.:BCX0283
CAS No.:1916734-50-3
- p-Hydroxyphenylethyl p-coumarate
Catalog No.:BCX0282
CAS No.:149030-02-4
- Trijugin A
Catalog No.:BCX0281
CAS No.:1233534-41-2
- Taxifolin 6-C-glucoside
Catalog No.:BCX0280
CAS No.:112494-39-0
- Pteroside T
Catalog No.:BCX0279
CAS No.:62043-51-0
- 5α,6α-Epoxy-3β-hydroxymegastigm-7-en-9-one
Catalog No.:BCX0292
CAS No.:50281-42-0
- Trijugin H
Catalog No.:BCX0293
CAS No.:1233534-55-8
- 24-O-Acetyllycoclavanol
Catalog No.:BCX0294
CAS No.:13956-53-1
- Racemoflavone
Catalog No.:BCX0295
CAS No.:106055-12-3
- Trijugin G
Catalog No.:BCX0296
CAS No.:1233534-53-6
- Isoorientin 3'-O-glucoside
Catalog No.:BCX0297
CAS No.:53098-95-6
- 2-Ethyl-3-methylmaleimide
Catalog No.:BCX0298
CAS No.:20189-42-8
- Balsaminone B
Catalog No.:BCX0299
CAS No.:213271-56-8
- Acutumidine
Catalog No.:BCX0300
CAS No.:18145-26-1
- Epiloliolide
Catalog No.:BCX0301
CAS No.:147730-52-7
- Yunnancoronarin C
Catalog No.:BCX0302
CAS No.:260406-45-9
- Tanshinol B
Catalog No.:BCX0303
CAS No.:189290-30-0
Studies Directed towards the Synthesis of the Acridone Family of Natural Products: Total Synthesis of Acronycines and Atalaphyllidines.[Pubmed:34693126]
ACS Omega. 2021 Oct 4;6(41):27062-27069.
A modular and flexible three-step synthetic strategy has been developed for the synthesis of acridone natural products of biological significance. The tetracyclic core of acridone derivatives has been achieved efficiently in high yield from commercially available anthranilic acid and phenol derivatives via condensation reaction, followed by regioselective annulation. Acridone alkaloids acronycine and noracronycine are synthesized in improved overall yields in fewer steps than the previously reported approaches. The method has further been used for the synthesis of atalaphyllidine and 5-Hydroxynoracronycine in excellent yields for the first time. Moreover, the synthetic utility of the present strategy has been showcased by the synthesis of oxa and thia analogues of acronycine alkaloid.
Can Antimalarial Phytochemicals be a Possible Cure for COVID-19? Molecular Docking Studies of Some Phytochemicals to SARS-CoV-2 3C-like Protease.[Pubmed:34376138]
Infect Disord Drug Targets. 2022;22(1):e290721195143.
OBJECTIVE: To evaluate the efficacy of reported anti-malarial phytochemicals as lead compounds for possible drug development against COVID-19. METHODS: An in silico approach was used in this study to determine through molecular docking the binding affinities and site of binding of these phytochemicals to the 3C-like protease of COVID-19 which is considered as the main protease of the virus. RESULTS: A number of anti-malarial phytochemicals like apigenin-7-O-glucoside, decurvisine, luteolin- 7-O-glucoside, sargabolide J, and shizukaols A, B, F, and G showed predicted high binding energies with DeltaG values of -8.0 kcal/mol or higher. Shizukaols F and B demonstrated the best binding energies of -9.5 and -9.8, respectively. The acridone alkaloid 5-Hydroxynoracronycine also gave a predicted high binding energy of -7.9 kcal/mol. CONCLUSION: This is for the first time that decursivine and several shizukaols were reported as potential anti-viral agents. These compounds merit further studies to determine whether they can be effective drug candidates against COVID-19.
The chemistry and biological activities of Citrus clementina Hort. Ex Tanaka (Rutaceae), a vegetatively propagated species.[Pubmed:32091235]
Nat Prod Res. 2021 Nov;35(22):4839-4842.
We report the chemistry and biological activities of a Cameroonian Citrus clementina Hort. Ex Tanaka, a vegetatively propagated species. The compounds isolated from this plant were determined to be the known 5-hydroxy-6,7,8,3',4'-pentamethoxyflavone (1), tangerine (3), nobilletin (4), 5,7,8,4'-tetramethoxyflavone (5), citracridone I (6), 5-Hydroxynoracronycine (7), citracridone III (8), xanthyletin (10), suberosin (9), E-suberenol (11), E-methoxysuberenol (13), 6-formylumbelliferone (12), aurantiamide acetate (2), limonin (14), stigmasterol, beta-sitosterol and beta-sitosterol-3-O-beta-D-glucoside. The structures of the compounds were established on the basis of their NMR spectroscopic data and comparison with published data. Methanol leaf extract and compounds 1, 2, 4, 6, 7 and 10 were evaluated for their anti-inflammatory, antioxidant, urease and anti-diabetic effects. Compound 10 showed antioxidant activity, anti-inflammatory effect, urease activity and anti-diabetic activity with IC(50) values of 47.3 microM, 33.5 microM, 25.2 microM and 33.9 microM respectively, values that were comparable to the respective positive standards.
Acridone alkaloids from the stem bark of Citrus aurantium display selective cytotoxicity against breast, liver, lung and prostate human carcinoma cells.[Pubmed:30189240]
J Ethnopharmacol. 2018 Dec 5;227:131-138.
ETHNOPHARMACOLOGICAL RELEVANCE: Citrus aurantium L. (Rutaceae) is used, either singly or as a part of a polyherbal preparation, in Nigerian traditional medicine for the management of cancer and inflammatory diseases. Currently, there is a dearth of knowledge demonstrating its anticancer potential. AIM OF THE STUDY: This study was carried out to determine the in vitro cytotoxicity of the crude extract of the stem bark of C. aurantium, identify and isolate the bioactive constituents and to establish the cytotoxicity of such constituents. MATERIALS AND METHODS: The powdered bark of C. aurantium was extracted by MeOH at room temperature (25-34 degrees C) and the crude extract was partitioned successively, with n-hexane, dichloromethane and methanol. Amongst the fractions, the DCM fraction was the most active and compounds were isolated from this fraction using a combination of chromatographic techniques. The structures of the isolated compounds were elucidated by spectroscopic means (MS, 1D and 2D NMR). The cytotoxicity of the extract, and the isolated compounds were evaluated by the MTT assay against four human cancer cell lines: A549 (lung), HepG2 (liver), MCF7 (breast) and PC3 (prostate). The selectivity of the isolated compounds was assessed using the normal human prostate epithelium cells (PNT2). RESULTS AND DISCUSSION: Of the three plant fractions, the DCM fraction showed significant cytotoxicity, with its highest activity against A549 cells (IC(50) = 3.88 microg/mL) and the least activity on HepG2 cells (IC(50) = 5.73 microg/mL). Six acridone alkaloids, citrusinine-I (1), citracridone-I (2), 5-Hydroxynoracronycine (3), natsucitrine-I (4), glycofolinine (5) and citracridone-III (6), were isolated from the DCM fraction of C. aurantium. The isolated compounds demonstrated potent to moderate cytotoxicity (IC(50) = 12.65-50.74 microM) against the cancer cells under investigation. It is noteworthy that the compounds exerted cytotoxicity at least four times more selective towards the carcinoma cells than the PNT2 cells. CONCLUSION: The results obtained from this study have provided some evidence for the ethnomedicinal use of C. aurantium against cancer and the acridone alkaloids present in its stem bark, have appeared to be responsible for the anticancer effects.
Structure and in vitro antiparasitic activity of constituents of Citropsis articulata root bark.[Pubmed:21985060]
J Nat Prod. 2011 Oct 28;74(10):2286-9.
From the results of an ethnomedicinal investigation of plants from Uganda with antimalarial activity, Citropsis articulata was selected because of the antiplasmodial effect of an ethyl acetate extract of its root bark. Thus, from the cyclohexane, ethyl acetate, and methanol extracts, two new heterocyclic compounds, omubioside (1) and katimborine (2), were isolated in addition to five known coumarins (rutarin (3), seselin (4), suberosin (5), demethylsuberosin (6), and haploperoside (7)), two known alkaloids (5-Hydroxynoracronycine (8) and 1,5-dihydroxy-2,3-dimethoxy-10-methyl-9-acridone (9)), trigonelline (10), and the limonoid 7alpha-obacunyl acetate (11). The best growth inhibitors of Plasmodium falciparum were alkaloids 8 and 9, with IC50 values of 0.9 and 3.0 mug/mL.
Anti-leishmanial activity of plant-derived acridones, flavaglines, and sulfur-containing amides.[Pubmed:21417924]
Vector Borne Zoonotic Dis. 2011 Jul;11(7):793-8.
Visceral and cutaneous leishmaniases are an important public health problem in endemic geographic regions in 88 countries worldwide, with around 12 million infected people. Treatment options are limited due to toxicity and teratogenicity of the available drugs, response problems in HIV/Leishmania co-infections, and upcoming resistances. In this study, we investigated the anti-leishmanial activity of 13 plant-derived compounds in vitro aiming to find new drug candidates. Toxicity of the compounds was evaluated in human primary hepatocytes, and hemolytic activity was examined in freshly isolated erythrocytes. Two acridones, 5-Hydroxynoracronycine and yukocitrine, two flavaglines, aglafoline and rocaglamide, and the sulfur-containing amide methyldambullin showed promising anti-leishmanial activities with 50% effective concentrations (EC50s) of 34.84, 29.76, 7.45, 16.45, and 6.29 muM, respectively. Hepatotoxic activities of 5-Hydroxynoracronycine, yukocitrine, and methyldambullin were significantly lower compared to miltefosine and lower or equal compared to artesunate, whereas the ones of rocaglamide and aglafoline were slightly higher compared to miltefosine and significantly higher compared to artesunate. None of the compounds showed hemolytic activity.
Bioactive acridone alkaloids from Swinglea glutinosa.[Pubmed:11575960]
J Nat Prod. 2001 Sep;64(9):1221-3.
A new prenylated acridone alkaloid, 1,3,5-trihydroxy-2,8-bis(3-methylbut-2-enyl)-10-methyl-9-acridone (1), was isolated from the stembark of Swinglea glutinosa, along with three known acridone alkaloids, 5-Hydroxynoracronycine (2), 1,3,5-trihydroxy-4-methoxy-2-(3-methylbut-2-enyl)-10-methyl-9-acridone (3), and 1,3,5-trihydroxy-4-methoxy-10-methylacridone (4). The isolated alkaloids were assessed in vitro against chloroquine-sensitive and -resistant Plasmodium falciparum strains and for cytotoxicity using HeLa cells.
Studies on the inhibitory effects of some acridone alkaloids on Epstein-Barr virus activation.[Pubmed:7480187]
Planta Med. 1995 Aug;61(4):366-8.
Twenty-five acridone alkaloids from Citrus plants were examined for their inhibitory effects on Epstein-Barr virus activation by a short-term in vitro assay. 5-Hydroxynoracronycine (20) and acrimarine-F (25) showed remarkable inhibitory effects.


