AcutumidineCAS# 18145-26-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18145-26-1 | SDF | Download SDF |
| PubChem ID | 442840 | Appearance | Powder |
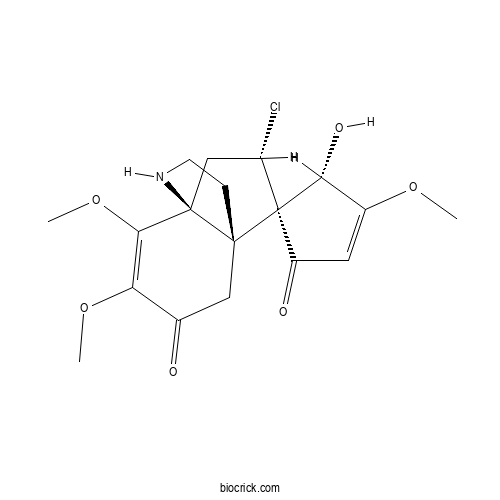
| Formula | C18H22ClNO6 | M.Wt | 383.8 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,4'S,6S,10R,11S)-11-chloro-4'-hydroxy-3',4,5-trimethoxyspiro[7-azatricyclo[4.3.3.01,6]dodec-4-ene-10,5'-cyclopent-2-ene]-1',3-dione | ||
| SMILES | COC1=CC(=O)C2(C1O)C(CC34C2(CCN3)CC(=O)C(=C4OC)OC)Cl | ||
| Standard InChIKey | SBALNGLYQFMKPR-NQTWQHAWSA-N | ||
| Standard InChI | InChI=1S/C18H22ClNO6/c1-24-10-6-12(22)18(14(10)23)11(19)8-17-15(26-3)13(25-2)9(21)7-16(17,18)4-5-20-17/h6,11,14,20,23H,4-5,7-8H2,1-3H3/t11-,14+,16+,17+,18+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Acutumidine Dilution Calculator

Acutumidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6055 mL | 13.0276 mL | 26.0552 mL | 52.1105 mL | 65.1381 mL |
| 5 mM | 0.5211 mL | 2.6055 mL | 5.211 mL | 10.4221 mL | 13.0276 mL |
| 10 mM | 0.2606 mL | 1.3028 mL | 2.6055 mL | 5.211 mL | 6.5138 mL |
| 50 mM | 0.0521 mL | 0.2606 mL | 0.5211 mL | 1.0422 mL | 1.3028 mL |
| 100 mM | 0.0261 mL | 0.1303 mL | 0.2606 mL | 0.5211 mL | 0.6514 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Balsaminone B
Catalog No.:BCX0299
CAS No.:213271-56-8
- 2-Ethyl-3-methylmaleimide
Catalog No.:BCX0298
CAS No.:20189-42-8
- Isoorientin 3'-O-glucoside
Catalog No.:BCX0297
CAS No.:53098-95-6
- Trijugin G
Catalog No.:BCX0296
CAS No.:1233534-53-6
- Racemoflavone
Catalog No.:BCX0295
CAS No.:106055-12-3
- 24-O-Acetyllycoclavanol
Catalog No.:BCX0294
CAS No.:13956-53-1
- Trijugin H
Catalog No.:BCX0293
CAS No.:1233534-55-8
- 5α,6α-Epoxy-3β-hydroxymegastigm-7-en-9-one
Catalog No.:BCX0292
CAS No.:50281-42-0
- 5-Hydroxynoracronycine
Catalog No.:BCX0291
CAS No.:27067-70-5
- Zedoalactone A
Catalog No.:BCX0290
CAS No.:170384-82-4
- 5-Deoxytrijugin C
Catalog No.:BCX0289
CAS No.:135971-83-4
- Zedoalactone B
Catalog No.:BCX0288
CAS No.:170384-81-3
- Epiloliolide
Catalog No.:BCX0301
CAS No.:147730-52-7
- Yunnancoronarin C
Catalog No.:BCX0302
CAS No.:260406-45-9
- Tanshinol B
Catalog No.:BCX0303
CAS No.:189290-30-0
- Spicatanol
Catalog No.:BCX0304
CAS No.:1158208-05-9
- (1S,16R)-1-Hydroxycryptotanshinone anhydride
Catalog No.:BCX0305
CAS No.:2301967-86-0
- Formononetin 7-O-β-D-apiofuranosyl-(1→6)-O-β-D-glucopyranoside
Catalog No.:BCX0306
CAS No.:857677-78-2
- Kushenol O
Catalog No.:BCX0307
CAS No.:102390-91-0
- Curzeone
Catalog No.:BCX0308
CAS No.:104068-56-6
- 16α-O-Methylneoquassin
Catalog No.:BCX0309
CAS No.:89498-94-2
- 16β-O-Ethylneoquassin
Catalog No.:BCX0310
CAS No.:343943-63-5
- 16α-O-Ethylneoquassin
Catalog No.:BCX0311
CAS No.:909272-59-9
- Methyl 2,4-dihydroxybenzoate
Catalog No.:BCX0312
CAS No.:2150-47-2
[Chemical constituents from rhizome of Menispermum dauricum and their anti-hypoxic activities].[Pubmed:30989885]
Zhongguo Zhong Yao Za Zhi. 2019 Feb;44(4):723-729.
To study the chemical constituents from the rhizome of Menispermum dauricum,fifteen compounds,N-methylcorydaldine( 1),thalifoline( 2),stepholidine( 3),acutumine( 4),daurisoline( 5),Acutumidine( 6),dauricicoline( 7),bianfugecine( 8),6-O-demethylmenisporphine( 9),bianfugedine( 10),dauricoside( 11),eleutheroside D( 12),aristolactone( 13),aristoloterpenateⅠ( 14) and aristolochic acid( 15) were isolated from 75% ethanol extract of Menispermi Rhizoma by using thin layer chromatography and column chromatography methods. Their structures were identified based on their physicochemical properties and spectral data. Among them,compounds 12-15 were obtained from the genus Menispermum for the first time. Six alkaloids with higher contents were subjected to evaluate the anti-hypoxic activities by using MTT method. As a result,six alkaloids exhibited significant anti-hypoxia activities.
A sensitive and selective UPLC-MS/MS method for simultaneous determination of 10 alkaloids from Rhizoma Menispermi in rat plasma and its application to a pharmacokinetic study.[Pubmed:26452875]
Talanta. 2015 Nov 1;144:662-70.
A sensitive and selective liquid chromatography-tandem mass spectrometry method has been developed and validated for simultaneous quantitation of 10 alkaloids (dauricine, daurisoline, N-desmethyldauricine, dauricicoline, dauriporphinoline, bianfugecine, dauricoside, stepholidine, acutumine and Acutumidine) from Rhizoma Menispermi in rat plasma. After addition of internal standard (verapamil), plasma samples were pretreated by a single-step protein precipitation with acetonitrile. Chromatographic separation was performed on a Waters BEH C18 column with gradient elution using a mobile phase composed of acetonitrile and water (containing 0.1% formic acid) at a flow rate of 0.3 mL/min. The analytes were detected without interference in the multiple reaction monitoring (MRM) mode with positive electrospray ionization. The validated method exhibited good linearity over a wide concentration range (r>/=0.9914), and the lower limits of quantification were 0.01-5.0 ng/mL for all the analytes. The intra-day and inter-day precisions (RSD) at three different levels were both less than 13.4% and the accuracies (RE) ranged from -12.8% to 13.5%. The mean extraction recoveries of analytes and IS from rat plasma were all more than 77%. The validated method was successfully applied to a comparative pharmacokinetic study of 10 alkaloids in rat plasma after oral administration of Rhizoma Menispermi extract.
Two new alkaloids and active anti-hepatitis B virus constituents from Hypserpa nitida.[Pubmed:17723297]
Bioorg Med Chem Lett. 2007 Oct 1;17(19):5316-20.
Two new alkaloids, hypserpanines A and B (1, 11), together with eleven known compounds, phenolbetain (2), acutumine (3), Acutumidine (4), dechloroacutumine (5), dauricumine (6), dauricumidine (7), pronuciferine (8), glaziovine (9), S-reticuline (10), magnoflorine (12) and laurifoline(13), were isolated from Hypserpa nitida Miers. (Menispermaceae) and chemically elucidated through spectral analyses. All the isolated alkaloids were evaluated for their anti-HBV activities in vitro using the HBV transfected Hep G2.2.15 cell line. The most active compound, dauricumidine (7), exhibited an IC(50) value of 0.450 mM (SI=4.13) on hepatitis B virus (HBV) surface antigen (HBsAg) secretion of the Hep G2.2.15 cell line.
Morphinane alkaloids with cell protective effects from Sinomenium acutum.[Pubmed:16038566]
J Nat Prod. 2005 Jul;68(7):1128-30.
One new morphinane alkaloid, sinomenine N-oxide (1), and one new natural occurring morphinane alkaloid, N-demethylsinomenine (2), together with six known alkaloids, 7,8-didehydro-4-hydroxy-3,7-dimethoxymorphinan-6-ol (3), sinomenine (4), sinoacutine (5), N-norsinoacutine, acutumine, and Acutumidine, were isolated from the stems of Sinomenium acutum. Their structures were elucidated on the basis of spectroscopic analysis and chemical methods. Compounds 2, 3, and 5 have protective effects against hydrogen peroxide-induced cell injury.
Alkaloids from Menispermum dauricum.[Pubmed:12377240]
Phytochemistry. 2002 Oct;61(4):439-42.
The alkaloids, dechloroAcutumidine and 1-epidechloroacutumine, together with three known alkaloids, Acutumidine, acutumine, and dechloroacutumine, were isolated from the rhizomes of Menispermum dauricum and their structures established by spectral and chemical methods. The cytotoxicity of each compound against the growth of human cell lines was studied, and acutumine selectively inhibited T-cell growth.
Chlorinated alkaloids in Menispermum dauricum DC: root culture.[Pubmed:11348110]
J Org Chem. 2001 May 18;66(10):3299-302.
Feeding experiments using (36)Cl showed that Menispermum dauricum root culture produces four alkaloids containing chlorine. They included the novel alkaloids dauricumine and dauricumidine as well as the known alkaloids acutumine and Acutumidine. The structures of novel alkaloids were established by spectroscopic, crystallographic, and chemical methods. These four alkaloids were labeled with (36)Cl, isolated, and fed independently to root cultures. Mutual conversion between acutumine and Acutumidine, and between dauricumine and dauricumidine by N-methylation and N-demethylation, was demonstrated. Moreover, dauricumine was converted to acutumine and Acutumidine. Epimerization of Acutumidine to dauricumidine or vice versa was not observed. These results suggest that dauricumine is the first chlorinated alkaloid formed in cultured M. dauricum roots. Skewed distribution of radioactivity derived from labeled dauricumine is proof that epimerization at C-1 proceeds at a lower rate than N-demethylation.


