Zedoalactone BCAS# 170384-81-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 170384-81-3 | SDF | Download SDF |
| PubChem ID | 15226640 | Appearance | Powder |
| Formula | C15H20O5 | M.Wt | 280.32 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
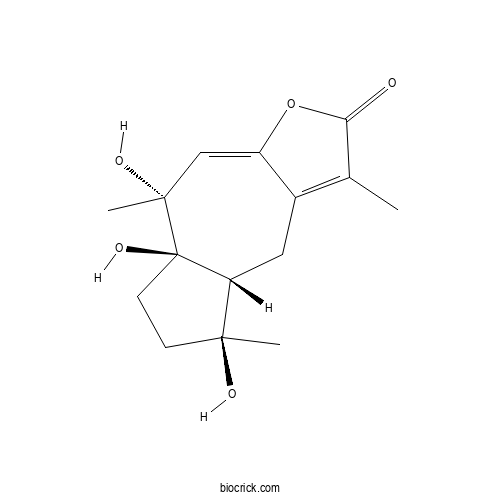
| Chemical Name | (5R,5aR,8S,8aR)-5,5a,8-trihydroxy-1,5,8-trimethyl-6,7,8a,9-tetrahydroazuleno[6,5-b]furan-2-one | ||
| SMILES | CC1=C2CC3C(CCC3(C(C=C2OC1=O)(C)O)O)(C)O | ||
| Standard InChIKey | HPNXJLIPUVXDNH-FAAHXZRKSA-N | ||
| Standard InChI | InChI=1S/C15H20O5/c1-8-9-6-11-13(2,17)4-5-15(11,19)14(3,18)7-10(9)20-12(8)16/h7,11,17-19H,4-6H2,1-3H3/t11-,13+,14-,15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Zedoalactone B Dilution Calculator

Zedoalactone B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5674 mL | 17.8368 mL | 35.6735 mL | 71.347 mL | 89.1838 mL |
| 5 mM | 0.7135 mL | 3.5674 mL | 7.1347 mL | 14.2694 mL | 17.8368 mL |
| 10 mM | 0.3567 mL | 1.7837 mL | 3.5674 mL | 7.1347 mL | 8.9184 mL |
| 50 mM | 0.0713 mL | 0.3567 mL | 0.7135 mL | 1.4269 mL | 1.7837 mL |
| 100 mM | 0.0357 mL | 0.1784 mL | 0.3567 mL | 0.7135 mL | 0.8918 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Zedoarolide B
Catalog No.:BCX0287
CAS No.:213833-36-4
- Trijugin B
Catalog No.:BCX0286
CAS No.:1233534-43-4
- Triumbelletin 7-O-glucoside
Catalog No.:BCX0285
CAS No.:2222584-03-2
- Ubine
Catalog No.:BCX0284
CAS No.:34469-09-5
- 3,4-Dihydroxy-N-(2-hydroxy-2-phenylethyl)benzamide
Catalog No.:BCX0283
CAS No.:1916734-50-3
- p-Hydroxyphenylethyl p-coumarate
Catalog No.:BCX0282
CAS No.:149030-02-4
- Trijugin A
Catalog No.:BCX0281
CAS No.:1233534-41-2
- Taxifolin 6-C-glucoside
Catalog No.:BCX0280
CAS No.:112494-39-0
- Pteroside T
Catalog No.:BCX0279
CAS No.:62043-51-0
- Aromadendrin 6-C-glucoside
Catalog No.:BCX0278
CAS No.:112494-34-5
- Trijugin C
Catalog No.:BCX0277
CAS No.:1233534-45-6
- Lup-20(29)-ene-2α,3α-diol
Catalog No.:BCX0276
CAS No.:55476-83-0
- 5-Deoxytrijugin C
Catalog No.:BCX0289
CAS No.:135971-83-4
- Zedoalactone A
Catalog No.:BCX0290
CAS No.:170384-82-4
- 5-Hydroxynoracronycine
Catalog No.:BCX0291
CAS No.:27067-70-5
- 5α,6α-Epoxy-3β-hydroxymegastigm-7-en-9-one
Catalog No.:BCX0292
CAS No.:50281-42-0
- Trijugin H
Catalog No.:BCX0293
CAS No.:1233534-55-8
- 24-O-Acetyllycoclavanol
Catalog No.:BCX0294
CAS No.:13956-53-1
- Racemoflavone
Catalog No.:BCX0295
CAS No.:106055-12-3
- Trijugin G
Catalog No.:BCX0296
CAS No.:1233534-53-6
- Isoorientin 3'-O-glucoside
Catalog No.:BCX0297
CAS No.:53098-95-6
- 2-Ethyl-3-methylmaleimide
Catalog No.:BCX0298
CAS No.:20189-42-8
- Balsaminone B
Catalog No.:BCX0299
CAS No.:213271-56-8
- Acutumidine
Catalog No.:BCX0300
CAS No.:18145-26-1
(1)H NMR-Based Metabolomics Approach Revealing Metabolite Variation of Black Turmeric (Curcuma caesia) Extracts and Correlation with Its Antioxidant and alpha-Glucosidase Inhibitory Activities.[Pubmed:37200789]
Food Technol Biotechnol. 2023 Mar;61(1):107-117.
RESEARCH BACKGROUND: Curcuma species (Zingiberaceae) are well known medicinal herbs in India and Southeast Asia. Despite various findings reporting their beneficial biological activities, very little information has been recorded on the Curcuma caesia. Thus, this study aims to determine the phenolic content, antioxidant and alpha-glucosidase inhibitory activity of both rhizome and leaves of C. caesia. EXPERIMENTAL APPROACH: Rhizome and leaves of C. caesia were dried with oven (OD) and freeze (FD)-drying methods, and extracted with different Phi(ethanol,water)=100:0, 80:20, 50:50 and 0:100. The bioactivities of C. caesia extracts were evaluated using in vitro tests; total phenolic content (TPC), antioxidant (DPPH and FRAP) and alpha-glucosidase inhibitory activity. Proton nuclear magnetic resonance ((1)H NMR)-based metabolomics approach was employed to differentiate the most active extracts based on their metabolite profiles and correlation with bioactivities. RESULTS AND CONCLUSIONS: The FD rhizome extracted with Phi(ethanol,water)=100:0 was observed to have potent TPC expressed as gallic acid equivalents, FRAP expressed as Trolox equivalents and alpha-glucosidase inhibitory activity with values of (45.4+/-2.1) mg/g extract, (147.7+/-8.3) mg/g extract and (265.5+/-38.6) microg/mL (IC(50)), respectively. Meanwhile, for DPPH scavenging activity, the Phi(ethanol,water)=80:20 and 100:0 extracts of FD rhizome showed the highest activity with no significant difference between them. Hence, the FD rhizome extracts were selected for further metabolomics analysis. Principal component analysis (PCA) showed clear discrimination among the different extracts. Partial least square (PLS) analysis showed positive correlations of the metabolites, including xanthorrhizol derivative, 1-hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)-(6E)-6-heptene-3,4-dione, valine, luteolin, zedoardiol, beta-turmerone, selina-4(15),7(11)-dien-8-one, Zedoalactone B and germacrone, with the antioxidant and alpha-glucosidase inhibition activities, whereas curdione and 1-(4-hydroxy-3,5-dimethoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)-(lE,6E)-1,6-heptadiene3,4-dione were correlated with alpha-glucosidase inhibitory activity. NOVELTY AND SCIENTIFIC CONTRIBUTION: C. caesia rhizome and leaf extracts contained phenolic compounds and had varies antioxidant and alpha-glucosidase inhibitory capacities. These findings strongly suggest that the rhizomes of C. caesia are an invaluable natural source of active ingredients for applications in pharmaceutical and food industries.
Bioactive chemical constituents from Curcuma caesia Roxb. rhizomes and inhibitory effect of curcuzederone on the migration of triple-negative breast cancer cell line MDA-MB-231.[Pubmed:31726856]
Nat Prod Res. 2021 Sep;35(18):3166-3170.
Rhizomes of Curcuma caesia are traditionally used to treat cancer in India. The aim is to isolate chemical constituents from C. caesia rhizomes through bioassay-guided fractionation. The extract, hexanes and chloroform fractions showed effect on MCF-7 and MDA-MB-231cells in cell viability assay. The chromatographic separation afforded germacrone (1), zerumbone (2), furanodienone (3), curzerenone (4), curcumenol (5), zederone (6), curcumenone (7), dehydrocurdione (8) from hexanes fraction and curcuminol G (9), curcuzederone (10), (1S, 10S), (4S,5S)-germacrone-1 (10), 4-diepoxide (11), wenyujinin B (12), alismoxide (13), aerugidiol (14), zedoarolide B (15), Zedoalactone B (16), zedoarondiol (17), isozedoarondiol (18) from chloroform fraction. This is first report of compounds 2, 9-13, 15-18 from C. caesia. The study demonstrated compounds 1-4 and 10 are the bioactive compounds. The effect of curcuzederone (10) on MDA-MB-231 cell migration showed significant inhibition in scratch and Transwell migration assays. The results revealed that curcuzederone could be a promising drug to treat cancer.
Antiinflammation constituents from Curcuma zedoaroides.[Pubmed:30109745]
Phytother Res. 2018 Nov;32(11):2312-2320.
Curcuma zedoaroides, a Zingiberaceae species, has been used for snake bite antidote and wound care in Thailand. Seven compounds were isolated from the bioactive chloroform extract consisted of one new guaiane sesquiterpene lactone, 5-epiphaeocaulisin A (4) and one new diarylheptanoid, 1,2,3,5-tetrahydroxy-1-(4-hydroxy-3-methoxyphenyl)-7-(4-hydroxyphenyl) heptane (7), together with five known guaiane-type sesquiterpene lactones including gweicurculactone (1), Zedoalactone B (2), phaeocaulisin C (3), zedoalactone H (5), and zedoalactone E (6). The antiinflammation was investigated on NO and TNF-alpha production using RAW264.7 cells. In addition, the expressions of genes involved in inflammation including iNOS, COX-2, and TNF-alpha were assessed. The results found that Compounds 1-7 presented their antiinflammation against NO production. The most potential effects were demonstrated on Compounds 1 and 7 with IC(50) of 27.3 and 32.6 muM, respectively. Although, Compounds 1 and 7 did not inhibit TNF-alpha production, their suppression on iNOS and COX-2 mRNA expression were revealed. These results support the ability of chloroform fraction, Compounds 1 and 7 on antiinflammation, whereas others exhibited moderate and mild effect.
Three new guaiane sesquiterpene lactones from rhizomes of Curcuma wenyujin.[Pubmed:23679107]
J Asian Nat Prod Res. 2013 Jul;15(7):723-30.
Three new guaiane sesquiterpene lactones (4S)-4-hydroxy-gweicurculactone (1), zedoalactone G (2), and (1R, 4R, 5S, 10S)-Zedoalactone B (3), and three known guaiane sesquiterpene lactones, including zedoarolide B (4), Zedoalactone B (5), and a new natural product (+)-zedoalactone A (6), were isolated from the rhizomes of Curcuma wenyujin Y.H. Chen et C. Ling. The structures were elucidated by spectroscopic methods including 1D and 2D NMR and HR-ESI-MS. The absolute configuration of 2 was determined via the calculated electronic circular dichroism (ECD), whereas the absolute configurations of 1 and 3 were determined via the ECD data of the [Rh2(OCOCF3)4] complex and [Mo2(OAc)4] complex, respectively. The inhibitory effects of compounds 1-6 on nitric oxide production in lipopolysaccharide-activated macrophages were evaluated. All of them exhibited weak anti-inflammatory activity.
Sesquiterpenes from Curcuma comosa.[Pubmed:18663560]
J Nat Med. 2009 Jan;63(1):102-4.
From the dried rhizomes of Curcuma comosa cultivating in Thailand, 26 known sesquiterpenes were isolated: zederone, zederone epoxide, furanodienone, isofuranodienone, 1(10)Z,4Z-furanodiene-6-one, glechomanolide, dehydrocurdione, neocurdione, curdione, 7 alpha-hydroxyneocurdione, 7 beta-hydroxycurdione, germacrone-1(10),4-diepoxide, germacrone, 13-hydroxygermacrone, curzerenone, curcolonol, alismol, alismoxide, zedoarondiol, isozedoarondiol, procurcumenol, isoprocurcumenol, aerugidiol, Zedoalactone B, curcumenone, and curcumadione. Their structures were elucidated on the basis of physicochemical evidence. Among them, glechomanolide, curzerenone, curcolonol, alismol, alismoxide, and zedoarondiol showed no significant optical activities, so they may be artifact products during the isolation or drying process.


